Photo AI
Last Updated Sep 24, 2025
Identifying Anions in Solution Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Identifying Anions in Solution quickly and effectively.
449+ students studying
Anion Identification
Introduction to Anion Identification
Definition and Role of Anions in Chemistry
- Anion: In chemistry, anions are negatively charged ions formed when an atom or molecule gains electrons, often involving non-metals.
- They are essential for balancing charges in ionic compounds, ensuring overall neutrality.
- Salt Formation: An example is the presence of chloride ions in sodium chloride.
- Charge Balancing: Crucial in maintaining stability and neutrality in compounds.
- pH Regulation: Anions affect the acidity or alkalinity of solutions through chemical reactions.
Anions significantly influence the reactivity and predictability of chemical reactions.
Importance of Identifying Anions
- Industrial Applications:
- Anion detection is vital in pharmaceuticals for ensuring medication purity and safety.
- In water treatment, it prevents corrosion and contamination.
- Environmental Contexts:
- Identifying nitrates in water samples is crucial for pollution control and environmental safety.
- Laboratory Uses:
- Accurate detection of anions is significant in titration experiments.
Recognising anions is essential for industrial safety and upholding environmental protection standards.
Overview of Qualitative Investigation Techniques
Core Techniques
- Flame Tests: Primarily applicable to cations, anions have limited characteristic flame colours.
- Precipitation Reactions: Effective for the formation of insoluble salts to identify anions.
- Complexation Reactions: Anions form detectable complexes with ligands, often colourful.
Ion Product: The product of the molar concentrations of ions in solution at a given point.
Ligands: Atoms or molecules donating a pair of electrons to a metal ion to form a complex.
Relevance of Tests for Specific Anions
- Chloride (Cl⁻): Detectable through the Silver Nitrate test, resulting in a white precipitate.
- Bromide (Br⁻) and Iodide (I⁻): Identifiable via halide tests with supporting chemical equations.
- Hydroxide (OH⁻): Engages in complexation with metals, with practical examples discussed.
- Acetate (CH₃COO⁻): Recognised by the distinct smell of vinegar.
- Carbonate (CO₃²⁻): Limewater tests demonstrate CO₂ release, such as .
- Sulfate (SO₄²⁻): Tested using Barium Chloride for water purity confirmation.
- Phosphate (PO₄³⁻): Discussed within the scope of precipitation and complexation reactions.
Solubility Product (Ksp) in Precipitation Reactions
- Theory of Solubility Product:
- Ksp represents the maximum ionic product before precipitation occurs.
- Precipitation occurs when the ion product exceeds Ksp.
Flame Tests for Indirect Anion Detection
Procedure for Conducting Flame Tests
- Preparation:
- Clean a wire loop using concentrated hydrochloric acid.
- Rinse thoroughly with distilled water.
- Sample Collection:
- Dip the loop into the sample solution or solid.
- Observation:
- Insert the loop into a Bunsen burner flame.
- Observe colour changes indicative of specific cations:
- Sodium: Yellow
- Barium: Green
Ensure accurate cleaning and rinsing to avoid contamination.

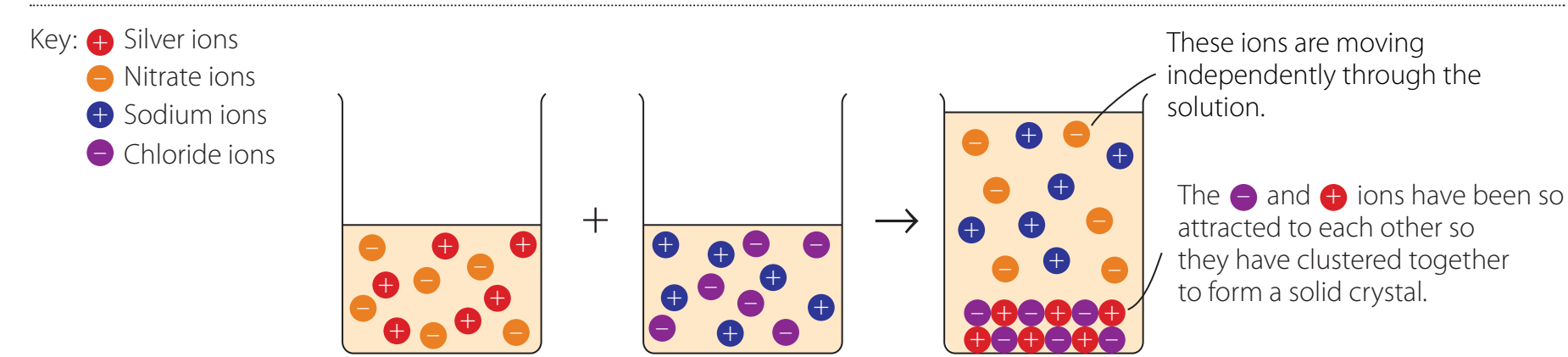
Precipitation Reactions
Detailed Explanation of Precipitation Process
-
Setup Process:
- Select appropriate reagents and prepare solutions accurately.
- Follow precise measurement to prevent contamination.
- Utilise sterilised instruments for accurate results.
-
Observations and Results:
- Visual Indicators:
- Different precipitates like AgCl (white), AgBr (cream), etc.
- Visual Indicators:
Complexation Reactions
Key Concepts
- Ligands and Coordination:
- Ligands: Act as electron pair donors.
- Coordination Number: The number of bonds a metal ion can form with ligands.
Example Reactions
- Hydroxide ions form complexes with metals like aluminium or iron.
Safety in Anion Identification
Required Personal Protective Equipment (PPE)
- Lab Coat
- Safety Goggles
- Gloves
- Fume Hood Access
PPE is the primary defence against chemical exposure.

Common Hazards
- Chemical Burns: From strong acids and bases.
- Inhalation Hazards: Volatile compounds.
- Skin Irritation: From corrosive substances.
Emergency Protocols
Immediate Actions:
- Eye Exposure: Rinse eyes with water for 15 minutes.
- Skin Contact: Clean thoroughly with soap and water.
- Inhalation: Move to fresh air and seek medical assistance if necessary.
Final Note on Lab Safety Guidelines
- Adherence to safety practices is non-negotiable.
Safety is paramount in all chemical work.
Through understanding these techniques and procedures, students and professionals can safely and accurately identify anions in various solution contexts. This foundation is critical for further applications in both academic and real-world scenarios.
500K+ Students Use These Powerful Tools to Master Identifying Anions in Solution For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
148 flashcards
Flashcards on Identifying Anions in Solution
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards21 quizzes
Quizzes on Identifying Anions in Solution
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes35 questions
Exam questions on Identifying Anions in Solution
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Identifying Anions in Solution
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Identifying Anions in Solution
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Identifying Anions in Solution you should explore
Discover More Revision Notes Related to Identifying Anions in Solution to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Analysis of Inorganic Substances
Environmental Monitoring Techniques
290+ studying
182KViews96%
114 rated
Analysis of Inorganic Substances
Identifying Ions in Solution
326+ studying
188KViews96%
114 rated
Analysis of Inorganic Substances
Analysis of Inorganic Substances
300+ studying
196KViews