Photo AI
Last Updated Sep 24, 2025
Atomic Structure Basics Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Atomic Structure Basics quickly and effectively.
204+ students studying
Atomic Structure Basics
An atom is the smallest unit of matter that retains the properties of an element. Understanding the arrangement and behaviour of subatomic particles is fundamental for mastering the basics of chemistry and predicting atomic interactions.
Fundamental Components of an Atom
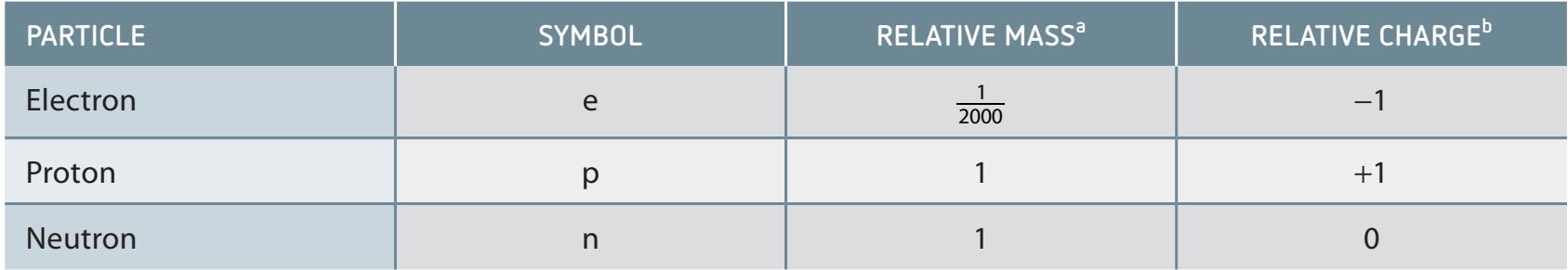
Atoms consist of three primary subatomic particles:
- Protons: Positively charged particles located within the nucleus.
infoNote
Proton: A positively charged particle essential for maintaining atomic stability.
- Neutrons: Neutral particles present in the nucleus that influence isotopic stability.
infoNote
Neutrons: Affect isotopic stability, located in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus, crucial in chemical reactions and bonding.
infoNote
Electron: Essential for chemical reactions and bonding.
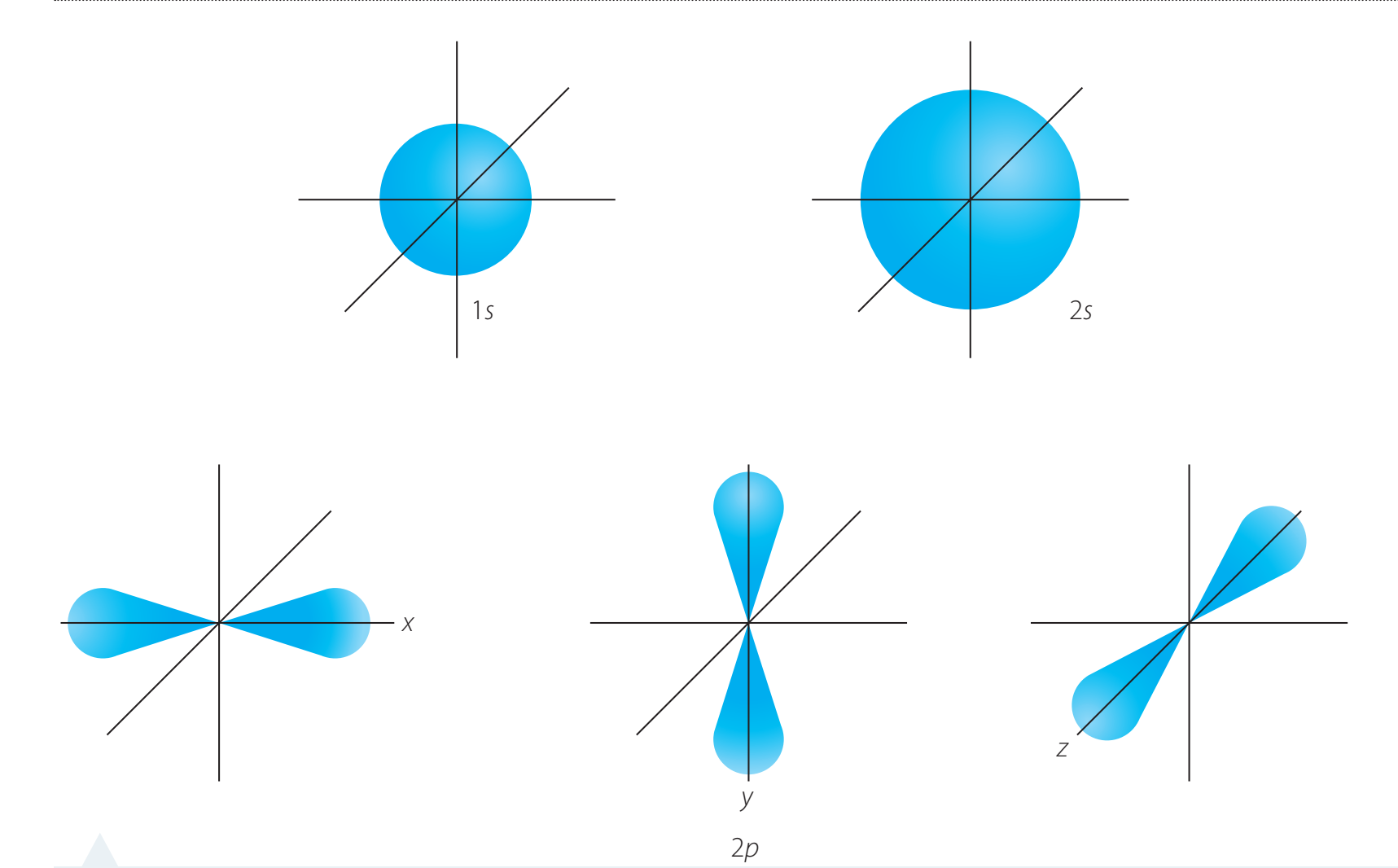
Visual Models of Atoms
Bohr vs Quantum Mechanical Model
-
Bohr Model: Represents electrons in fixed orbits, akin to a solar system.
-
Quantum Mechanical Model: Describes electrons as existing in orbitals, which are regions of probability.

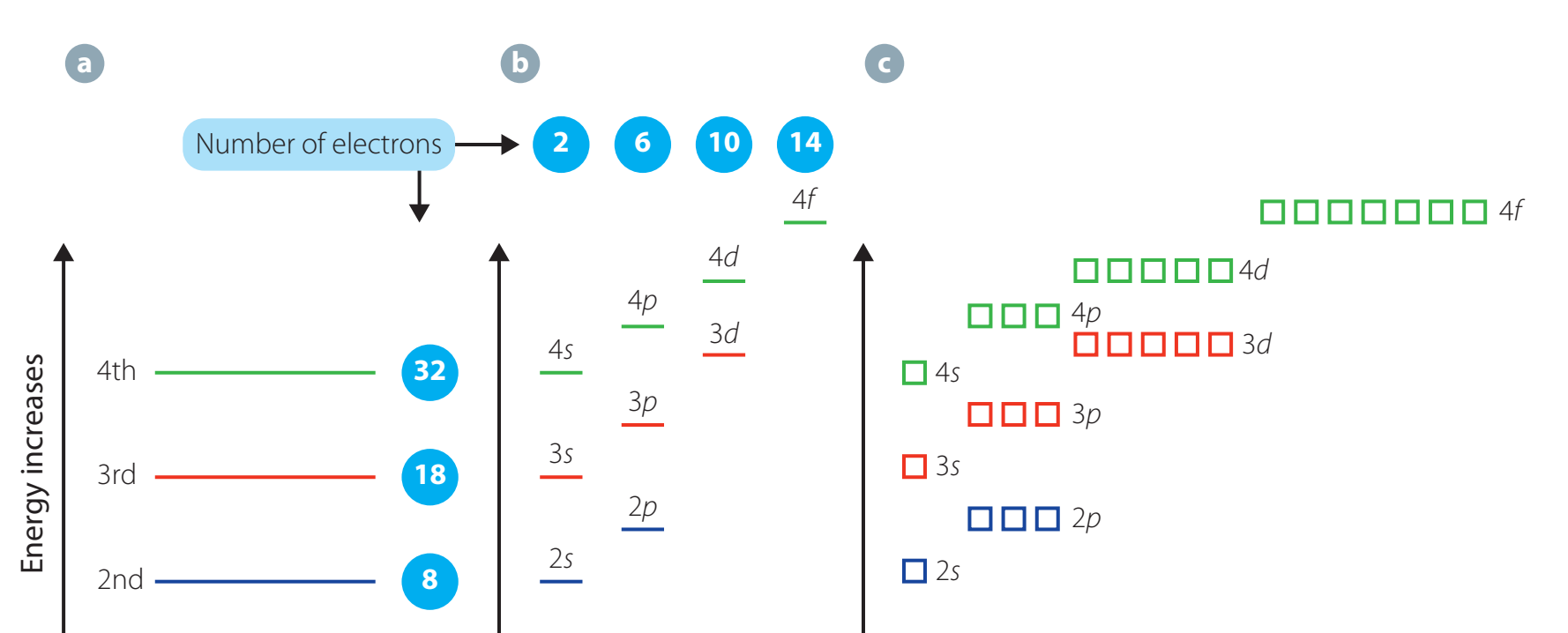
Electron Shells, Subshells, and Orbital Notation
Introduction to Electron Shells and Subshells
-
Shells & Subshells: Determine an atom's reactivity and potential for bond formation.
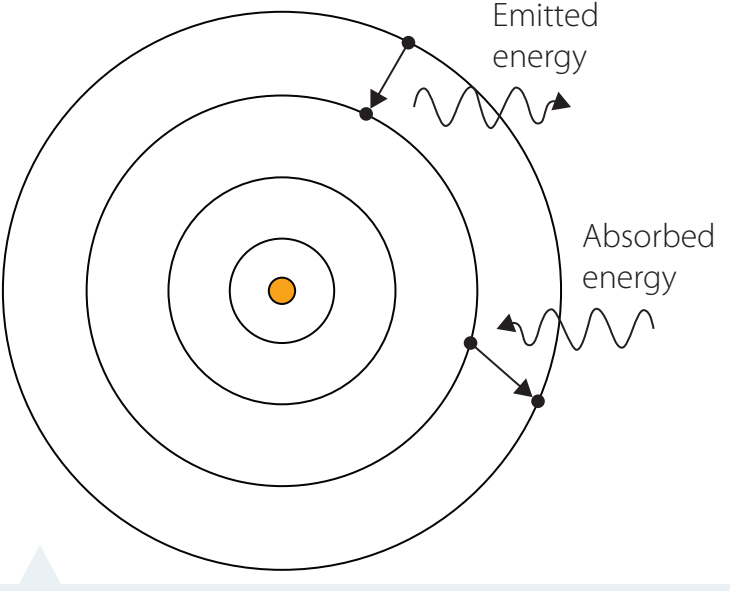
Understanding Energy Levels and Discreteness
-
Discrete Energy Levels: Atoms possess specific energy states as opposed to a continuous spectrum.

Electrons can only occupy specific energy levels; they do not travel in fixed paths.
Isotopes and Their Properties
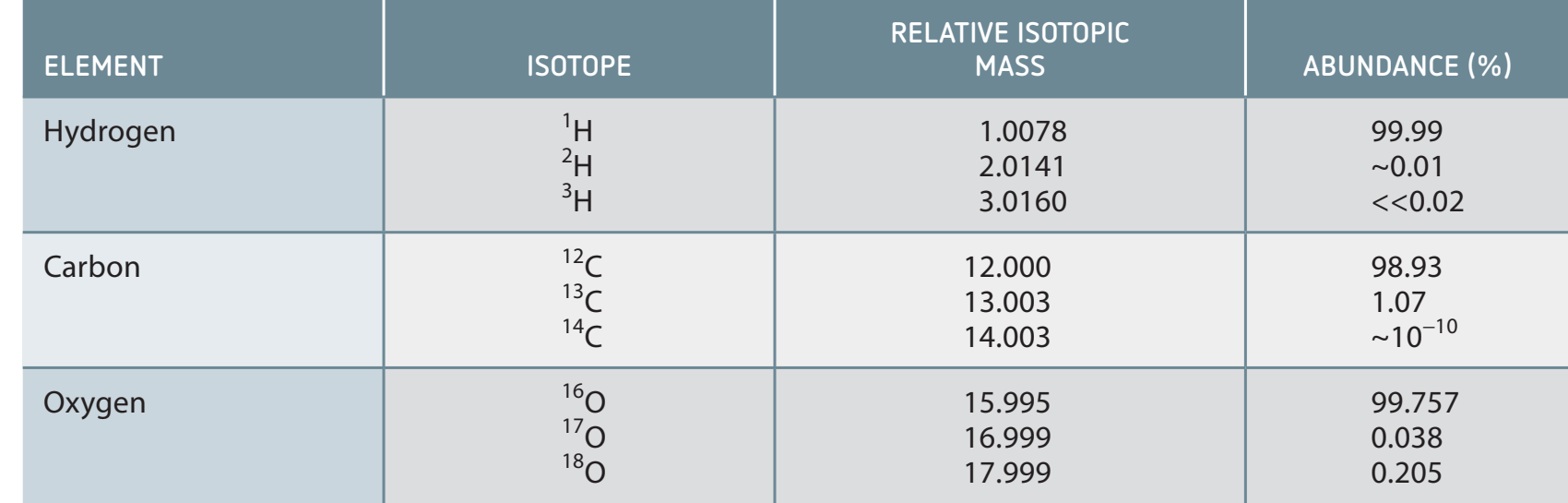
Isotopes: Definition and Characteristics
Isotopes: Variants of atoms with the same number of protons but differing numbers of neutrons.
- Exhibit identical chemical properties, with differences affecting mass and density.
- Examples include both stable (Carbon-12) and unstable isotopes (Carbon-14).
Representing Isotopes
-
Isotopes are represented using scientific notation:
- Example: Carbon-12 as .
Electron Configuration Principles
Key Principles
-
Aufbau Principle: Electrons populate energy levels from lowest to highest.
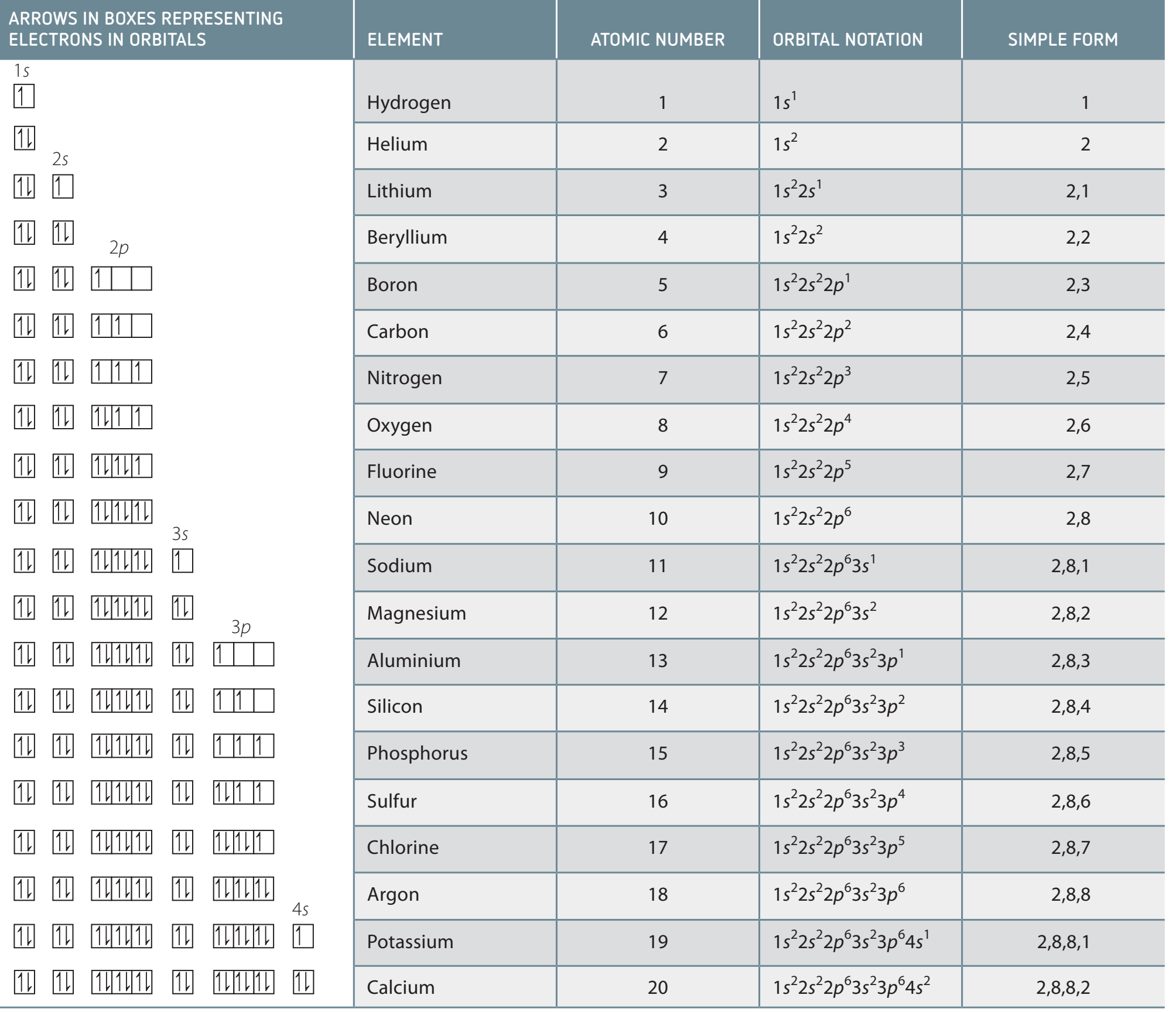
-
Hund's Rule & Pauli Exclusion: Determines the arrangement of electron pairs and the uniqueness of their spins.
Electron Transitions and Spectra
-
Ground vs. Excited State: Describes how electrons absorb or emit energy when transitioning between energy levels.
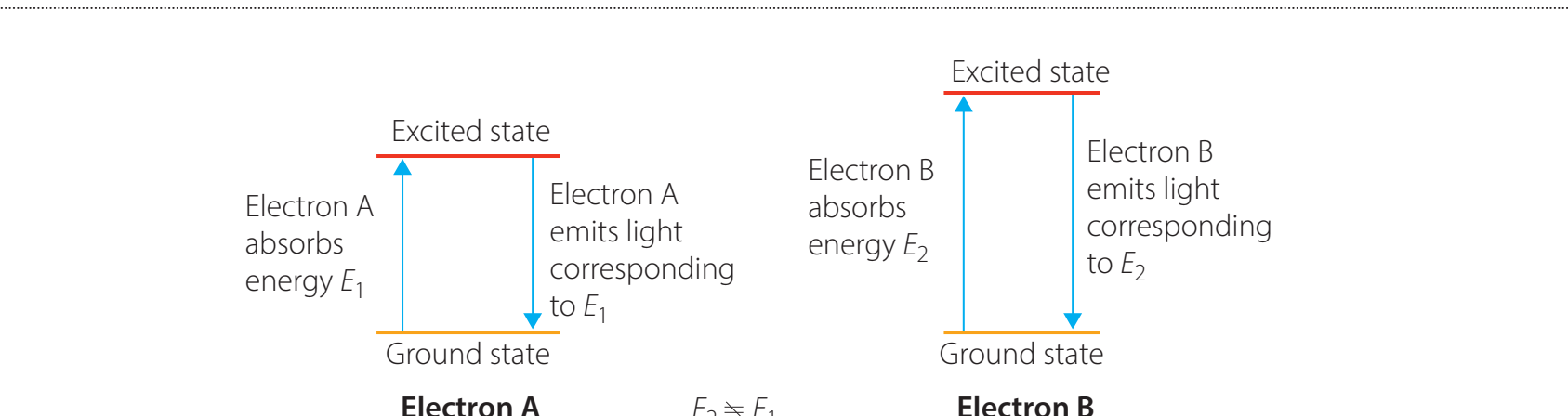
-
Emission & Absorption Spectra: Unique to each element, akin to fingerprints.
Orbital Notation
Orbital Notation Basics
-
Orbital Notation: Provides a visual representation of electron configurations and spin orientation.

-
Key Principles:
- Hund's Rule: Indicates filling unoccupied orbitals before pairing.
- Pauli Exclusion Principle: States that electrons in the same orbital must have opposite spins.
Overall, a profound understanding of atomic structure and notations—such as spdf and orbital diagrams—enriches comprehension of chemical behaviours and properties, forming the foundation of modern chemistry.
500K+ Students Use These Powerful Tools to Master Atomic Structure Basics For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
423 flashcards
Flashcards on Atomic Structure Basics
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards31 quizzes
Quizzes on Atomic Structure Basics
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes14 questions
Exam questions on Atomic Structure Basics
Boost your confidence with real exam questions.
Try Chemistry Questions3 exams created
Exam Builder on Atomic Structure Basics
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Atomic Structure Basics
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Atomic Structure Basics you should explore
Discover More Revision Notes Related to Atomic Structure Basics to Deepen Your Understanding and Improve Your Mastery
