Photo AI
Last Updated Sep 24, 2025
Atomic Structure Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Atomic Structure quickly and effectively.
266+ students studying
Atomic Structure
Introduction to Isotopes
Definitions and Basic Concepts
- Isotopes: Different forms of the same element that have an equal number of protons but different numbers of neutrons.
Isotopes: Different forms of an element.
- Identical atomic numbers: Same number of protons.
- Varied mass numbers: Due to different neutron counts.
Stability of Isotopes
- Stable Isotopes: These remain unchanged over time and are non-radioactive.
- Example: Oxygen-16.
- Unstable Isotopes (Radioisotopes): These experience radioactive decay.
- Example: Carbon-14, used in radiocarbon dating to date ancient artefacts in archaeology.
Visualise stability as a seesaw. A balanced seesaw represents stability (e.g., equal weight on both ends), whereas a tilt signifies instability.
- Neutron-to-Proton Ratio: Acts as a balance, keeping the seesaw level.
Zone of Stability
- Zone of Stability:
- Describes stable neutron-to-proton ratios.
- Important for predicting isotope behaviour, impacting nuclear safety and research.
Understanding the zone of stability aids in predicting whether an isotope is stable or radioactive.
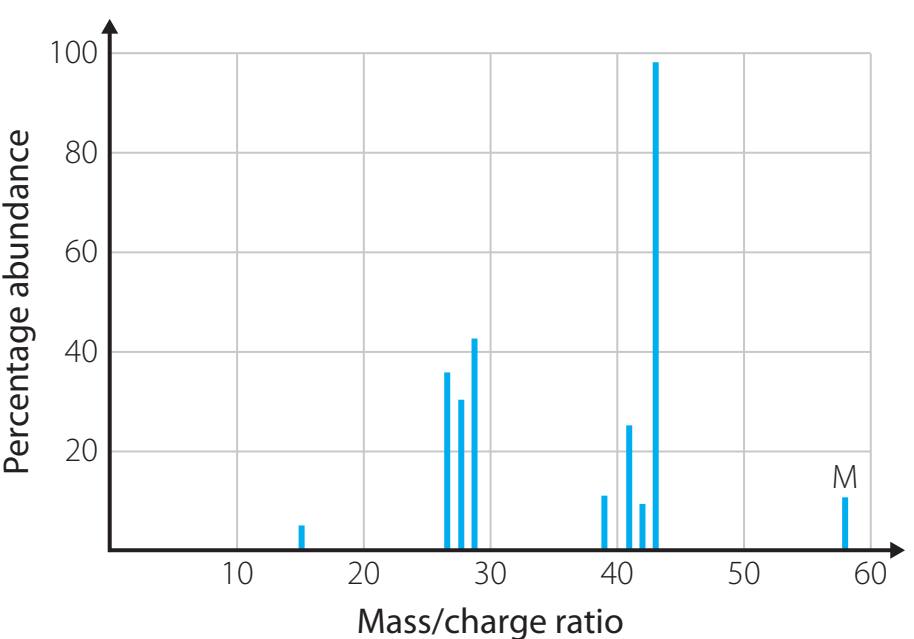
Common Examples
- Hydrogen Isotopes:
- Protium: Stable with no neutrons.
- Deuterium: Stable with one neutron, used in heavy water.
- Tritium: Unstable and radioactive, with two neutrons, found in glow-in-the-dark items.
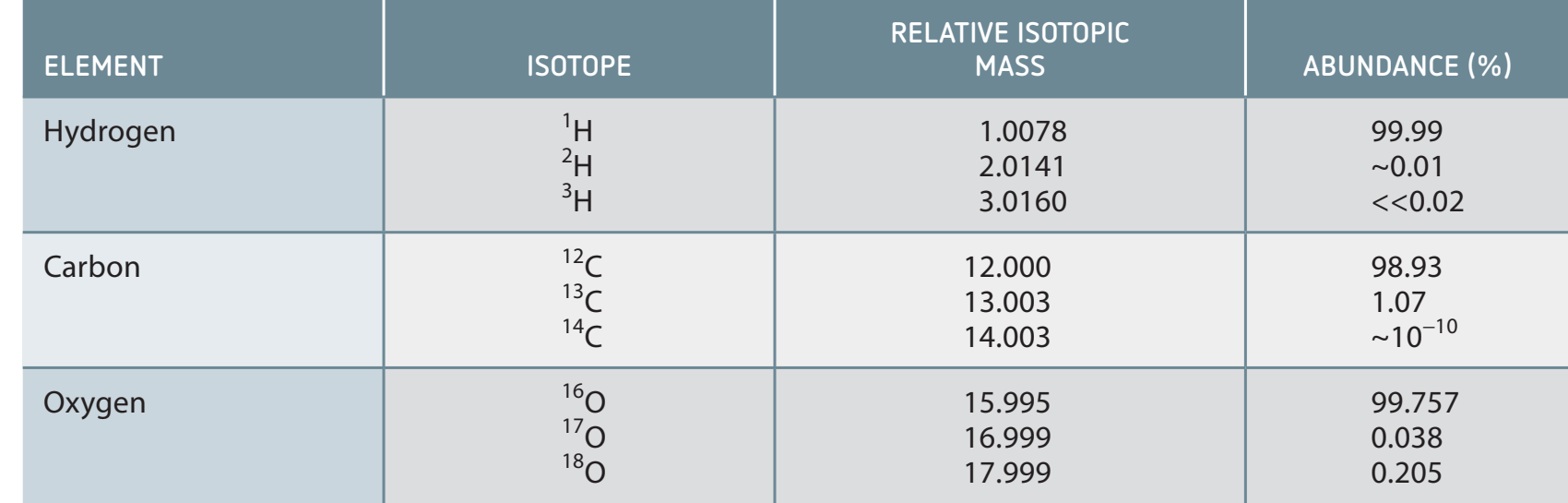
Addressing Misconceptions
- Misconception: Isotopes display unique chemical behaviours due to mass differences.
- Clarification: Chemical properties are primarily governed by electron arrangement.
- Analogy: A gadget's function depends on its circuit design, not its size, similar to how electron configurations dictate atomic behaviour.
Introduction to Isotopic Notation
Isotopic notation is a symbolic tool for identifying isotopes of elements. It underscores differences in atomic structure and mass, crucial for uses such as medical diagnostics and radiocarbon dating. For instance, Carbon-14 aids in dating ancient artefacts with precision.
Definition
Isotopic notation: A system for differentiating isotopes by mass and atomic numbers.
Detailed Explanation
Isotopic notation is represented as :
- A (mass number): Total count of protons and neutrons.
- Z (atomic number): Count of protons within the atom.
- M (element symbol): Chemical symbol of the element.
Importance
- Changes in neutron count impact stability while preserving chemical properties.
Examples and Breakdown
Chlorine Isotopes
- :
- Mass number = 35
- Atomic number = 17
- Element symbol = Cl
- :
- Mass number = 37
- Atomic number = 17
- Element symbol = Cl
Both isotopes share the same atomic number but differ in mass due to neutron variation.
Carbon Isotopes
- - Standard form of carbon.
- - Essential for radiocarbon dating, determining the age of organic materials.
Step-by-Step Breakdown
- Begin by identifying the atomic number (Z).
- Derive the mass number (A) using the sum of protons and neutrons.
- Apply isotopic notation by combining these values.
Visual and Diagrammatic Representation

- Diagram Elements:
- Review the labeled "A" and "Z" numbers to discern isotopic differences.
- Observe how elements retain their identity despite neutron differences.
Significance in Nuclear Reactions
Isotopic notation is significant in monitoring isotopic changes during nuclear reactions:
- Aids in tracking isotope transformations during reactions.
- Provides insight into reaction mechanisms and element conservation.
Addressing Misunderstandings
- Misconception: 'Isotopes are different elements due to mass difference.'
• Reality:
- Isotopes have the same number of protons.
- They vary only in neutron count, not in elemental type.
Introduction and Definitions
-
Atomic Number (Z): Number of protons in an atom's nucleus, determining the element's identity.
- Role: Essential for chemical identity, as all atoms of an element share the same atomic number.
-
Mass Number (A): Sum of protons and neutrons.
- Often referred to as the nucleon number.
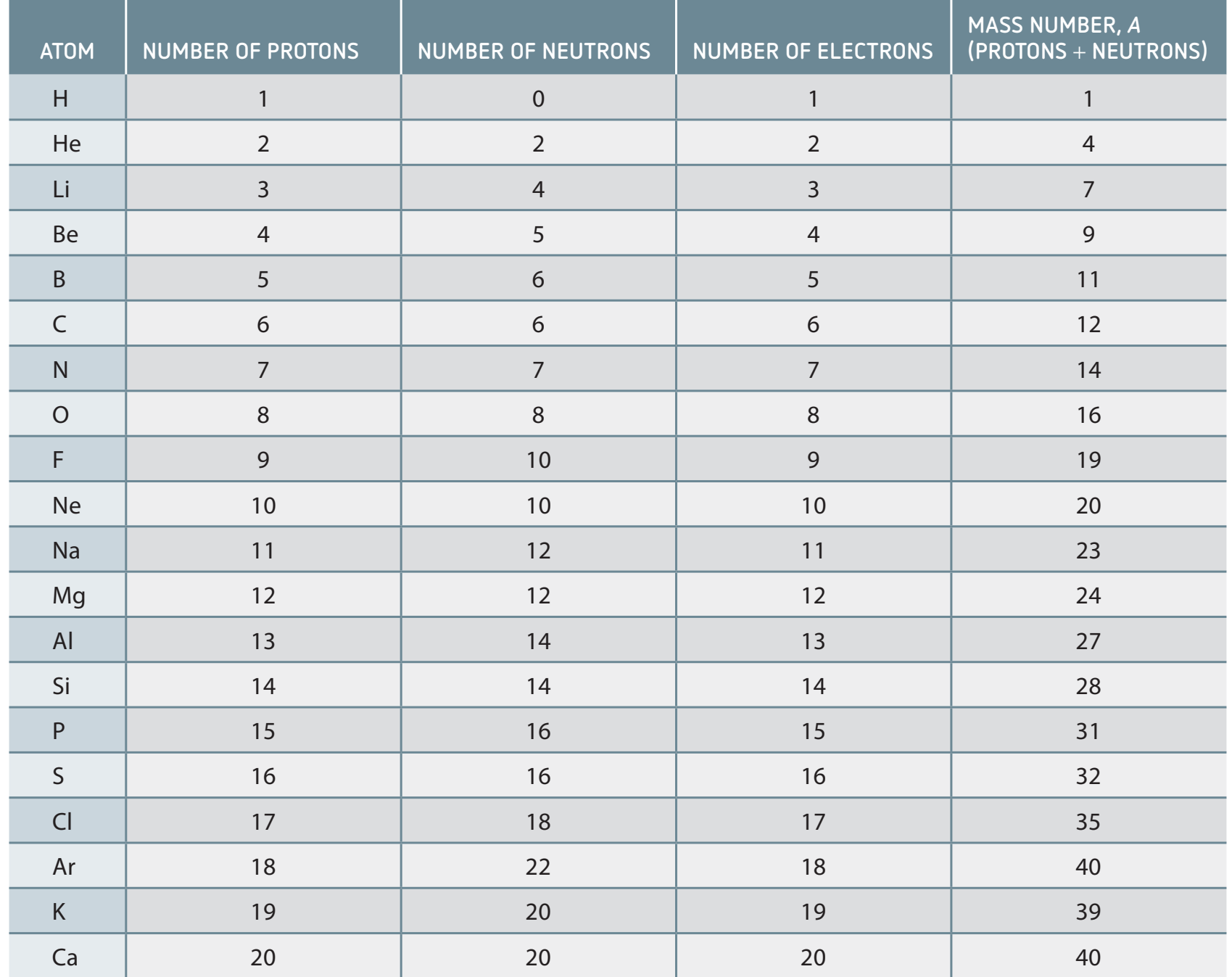
An element sharing the same atomic number belongs to the same category.
Example
- Carbon Isotopes:
- Carbon-12: ,
- Carbon-13: ,
These isotopes have equal protons but different total mass due to varied neutrons.
Role and Impact on Elemental Identity
-
Atomic Number: Determines each element's identity and chemical properties.
-
Mass Number: Influences physical properties but not chemical identity.
-
Comparison of Influence:
- Atomic Number:
- Defines the element—Does not change.
- Ensures consistent chemical properties.
- Mass Number:
- Alters physical properties, notably in isotopes.
- Atomic Number:
While the atomic number ensures chemical consistency, variations in mass number lead to differences in physical characteristics among isotopes.
Practical Exercises
Neutron Calculation Formula
-
Formula:
- Number of Neutrons =
-
A visual aid can provide better comprehension.
- Example 1 - Sodium-23
- Identify:
- Mass Number ()
- Atomic Number ()
- Calculate:
- Neutrons =
- Reflect:
- Knowing the neutron count aids in understanding atomic differences.
- Example 2 - Neon-20
- Identify:
- Mass Number ()
- Atomic Number ()
- Calculate:
- Neutrons =
Precise neutron calculations clarify isotope identities.
Engagement via Visual Tools
- Utilise the periodic table to observe how elements are arranged by atomic number.
Clarification of Misconceptions
- Misconception: Different isotopic mass leads to varying chemical properties.
- Reality: Chemical properties do not vary because the atomic number is unchanged.
Understanding misconceptions and their corrections is key to mastering elemental chemistry concepts.
Integration of Diagrams
- Diagrams clarify isotopic contrasts and highlight how atomic and mass numbers correlate.
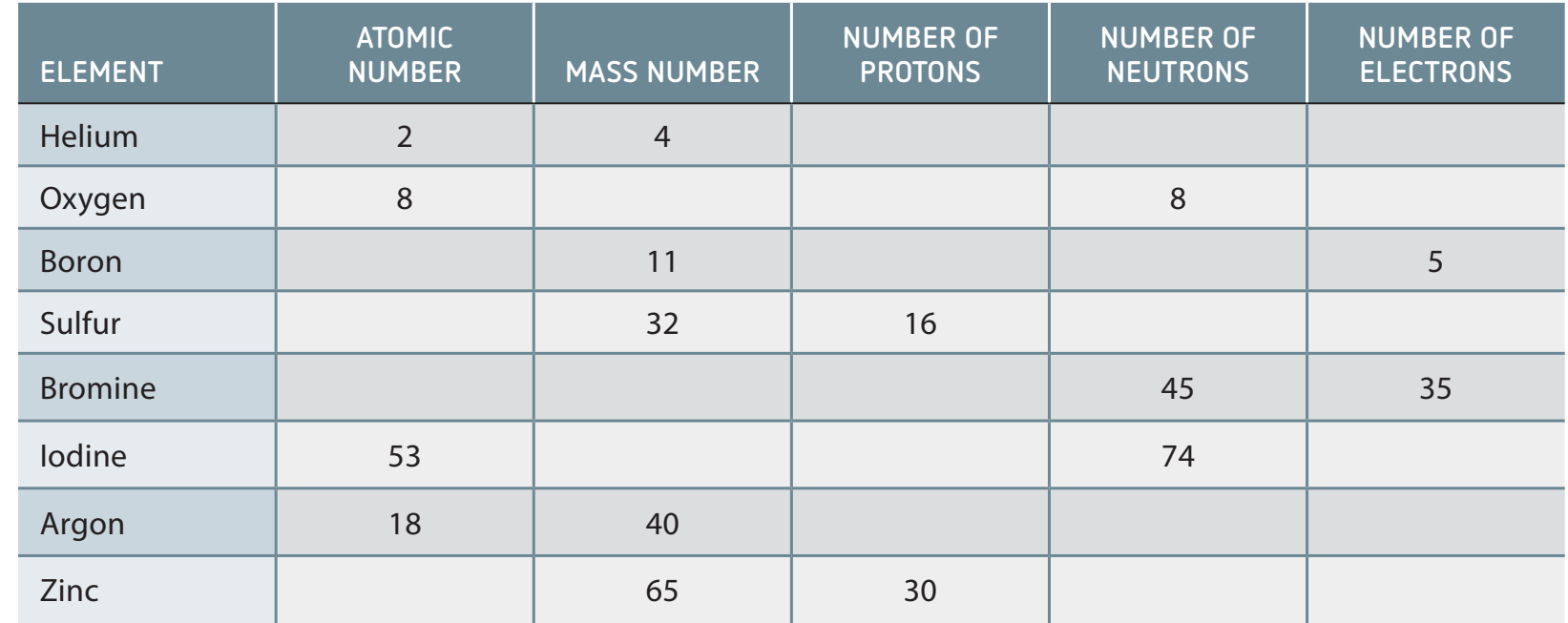
- Annotated Table:
SPDF Notation
Structure of SPDF Notation
-
SPDF Notation: SPDF notation describes the electronic configuration of atoms by distributing electrons among energy sublevels called s, p, d, and f orbitals, key to understanding energy levels and subshells.
- s-Orbital: Spherical, lowest energy level.
- p-Orbital: Dumbbell-shaped, has higher energy than s.
- d-Orbital: More intricate shapes appearing in transition metals.
- f-Orbital: Most complex, highest energy seen in lanthanides and actinides.
-
Energy Levels and Subshells:
- Energy Levels (n): Indicate principal quantum number, reflecting electron energy.
- Subshells (s, p, d, f): Define the shape and chemical properties of the orbital.
Energy Sublevels and Principles
-
Aufbau Principle: Outlines electron filling order, starting from the lowest energy.
- Example: Helium adheres to this with .
-
Hund's Rule: Electrons fill each orbital singly before pairing.
- Example: Carbon with initially no paired 2p electrons.
-
Pauli Exclusion Principle: No two electrons in an orbital can have identical quantum numbers.
- Example: Neon reflects this principle.
Recap of Key Principles
- Aufbau indicates: Lower energy levels are filled first.
- Hund's rule mandates: Single occupancy per orbital before any pairing.
- Pauli exclusion prevents: Identical quantum numbers for two electrons.
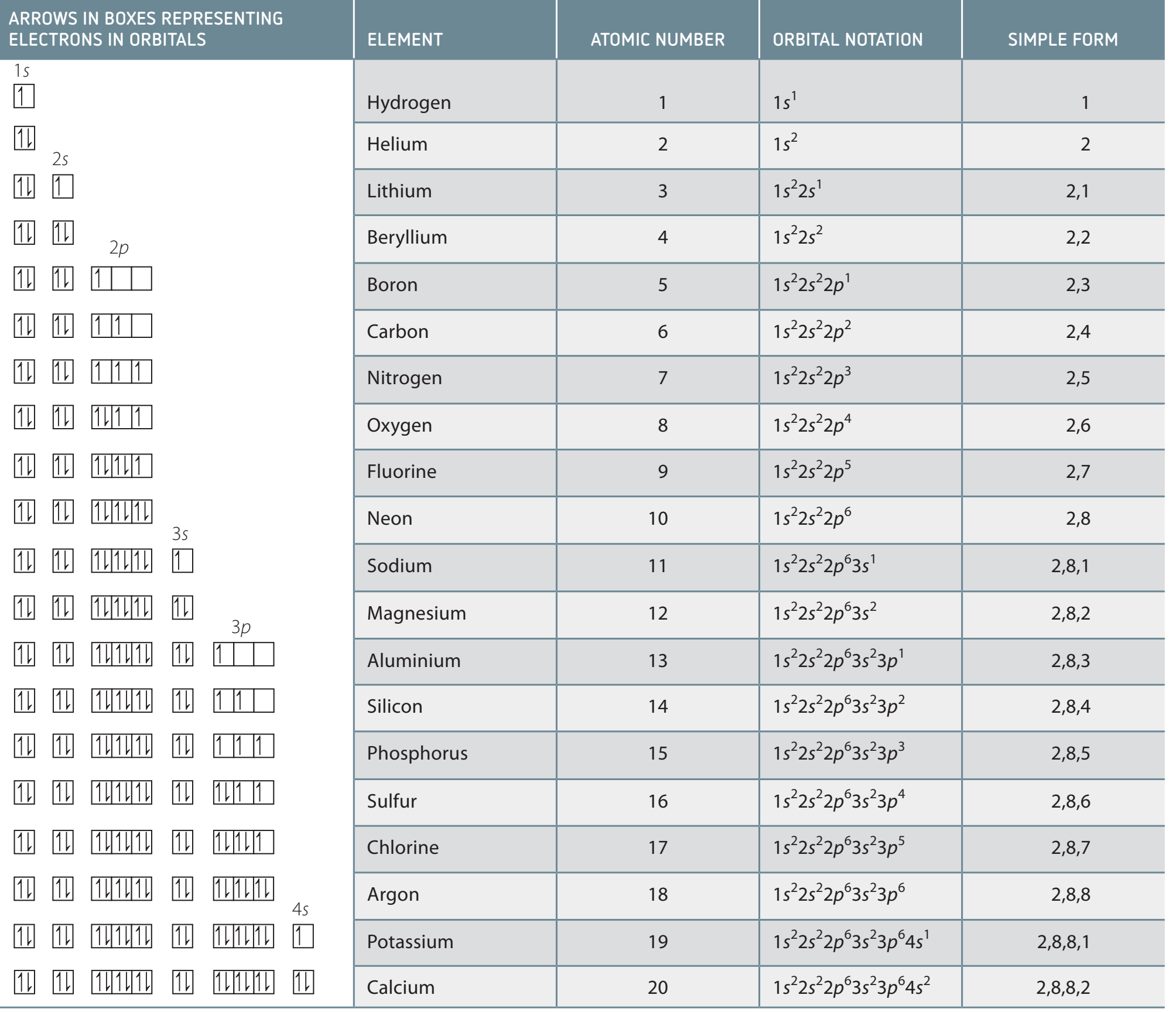
Diagram Caption: Examine how electrons fill in accordance with the Aufbau principle shown here.
500K+ Students Use These Powerful Tools to Master Atomic Structure For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
423 flashcards
Flashcards on Atomic Structure
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards31 quizzes
Quizzes on Atomic Structure
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes14 questions
Exam questions on Atomic Structure
Boost your confidence with real exam questions.
Try Chemistry Questions3 exams created
Exam Builder on Atomic Structure
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Atomic Structure
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Atomic Structure you should explore
Discover More Revision Notes Related to Atomic Structure to Deepen Your Understanding and Improve Your Mastery
