Photo AI
Last Updated Sep 24, 2025
Atomic Sublevels Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Atomic Sublevels quickly and effectively.
267+ students studying
Atomic Sublevels
Introduction
Have you ever considered how the elements' arrangement in the periodic table corresponds to their properties? The key lies in the sublevel filling principles. These principles are the rules governing electron configurations within atomic orbitals. Understanding these rules is crucial for comprehending the periodic table's organisation.
Understanding Principal Energy Levels
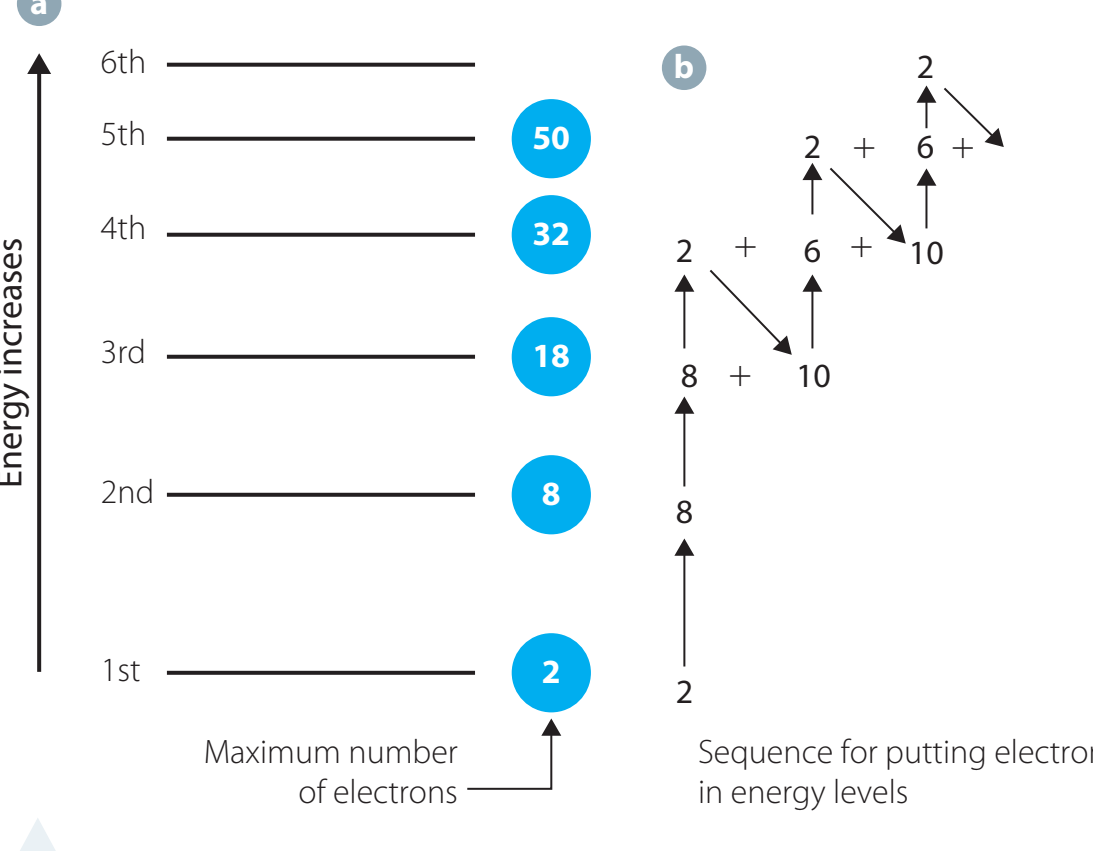
Principal Energy Levels (PELs) are determined by the quantum number and indicate an electron's proximity to the nucleus within an atom. Here is what is important to know:
- A higher implies that electrons are located further from the nucleus, and the level possesses more energy.
- Visualised as "concert halls", larger indicates larger halls with more complex electron 'arrangements'.
- Fundamental in understanding how elements interact chemically and align in the periodic table.
Principal Energy Levels:
- Represented by
- Higher values denote increased energy
- Essential for understanding chemical behaviour
Diving into Sublevels
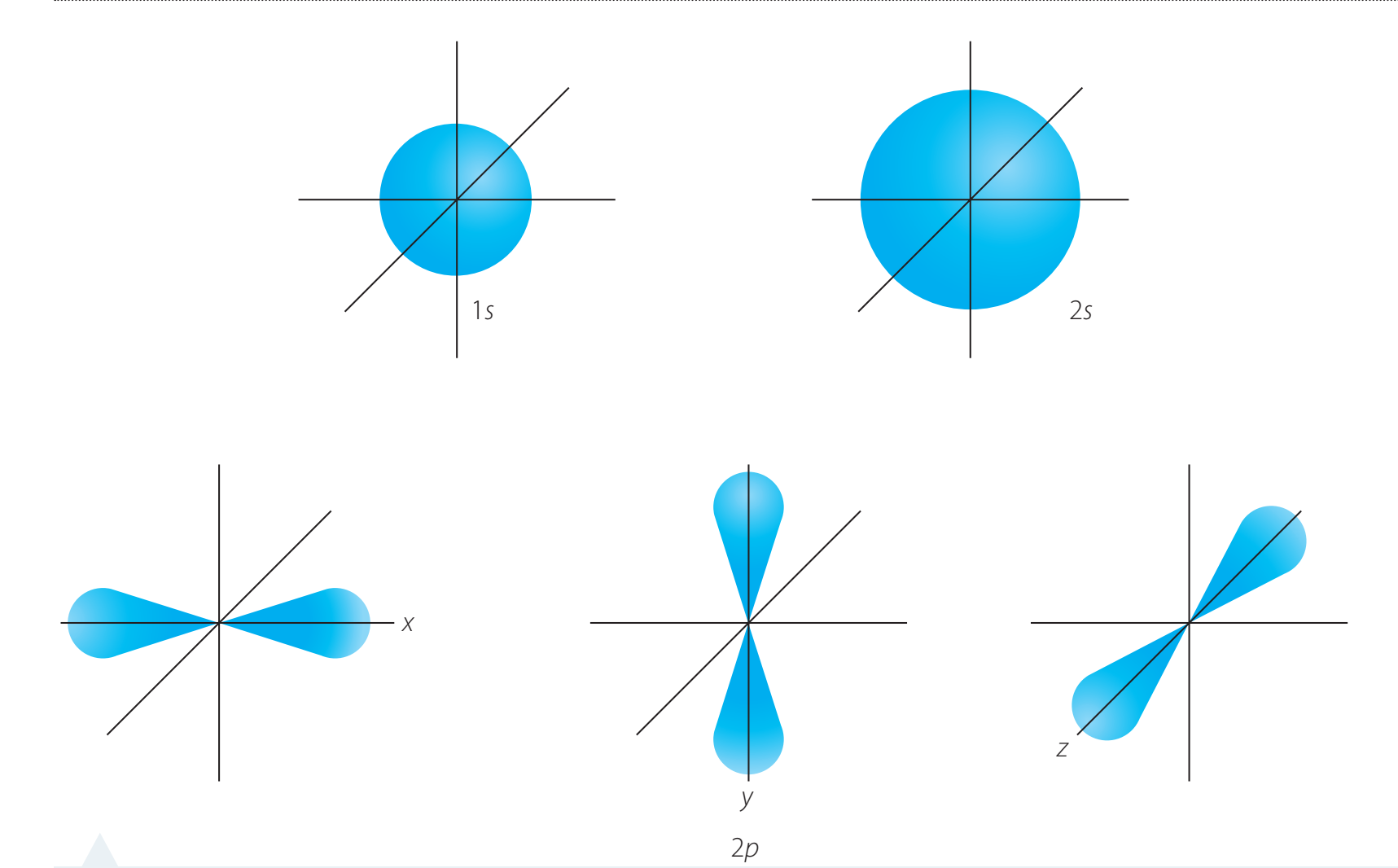
Sublevels refine PELs into more specific sections, labelled as 's', 'p', 'd', and 'f'. These sublevels define how electrons are organised:
- s Sublevel: Contains 1 orbital and can accommodate up to 2 electrons.
- p Sublevel: Consists of 3 orbitals, with a capacity of 6 electrons.
- d Sublevel: Composed of 5 orbitals, supporting up to 10 electrons.
- f Sublevel: Encompasses 7 orbitals, with space for 14 electrons.
Extended Analogy: Think of each sublevel as a "room" in a concert hall with designated seating (orbitals), organising the electron audience. This analogy aids in visualising electron distribution.
Overview of Sublevel Characteristics
-
Shape and Geometry:
- s sublevel: spherical
- p sublevel: Dumbbell-shaped
- d sublevel: Cloverleaf or complex
- f sublevel: Intricate
-
Number of Orbitals and Electron Capacity:
| Sublevel | Number of Orbitals | Maximum Electron Capacity |
|---|---|---|
| s | 1 | 2 |
| p | 3 | 6 |
| d | 5 | 10 |
| f | 7 | 14 |
Quantum Numbers and Sublevels
Principal Quantum Number (n)
- Definition: Specifies the principal energy level of an electron.
- Significance:
- Determines the energy shell.
- Closest proximity to the nucleus when .
Principal Quantum Number (n): Indicates the energy level and proximity of electrons to the nucleus.
Angular Momentum Quantum Number (l)
- Definition: Describes the shape of the orbital.
- Significance:
- Defines the sublevel (s, p, d, f).

Predictability in Chemical Behaviour
Predicting chemical behaviour, including reactions, requires knowledge of electron arrangements:
- Hund's Rule: Electrons fill open orbitals individually before pairing. This demonstrates energy efficiency.
Understanding Electron Configuration:
- Predicts material reactivity
- Informs bonding processes
Aufbau Principle
- Definition: Aufbau Principle: Electrons occupy the lowest available energy levels first.
Detailed Process
- Step-by-Step Explanation:
- Apply n+l Rule: Determine sublevel energy using .
- Filling Order: Sublevels are completed in order of increasing .
Worked Example: To determine the electron configuration of carbon (atomic number 6):
- Start with the lowest energy sublevel (1s)
- Fill each sublevel in order: 1s², 2s², 2p²
- The final 2 electrons go into the 2p sublevel, following Hund's rule (one electron in each of two 2p orbitals)
Therefore, carbon's electron configuration is 1s² 2s² 2p².
Significance of These Principles
- Predictability: These principles form a framework for predicting the properties and interactions of elements.
- Structural Significance:
- Predicts chemical bonding patterns.
- Relates to trends observed in the periodic table.
- Exceptions: Transition elements exhibit unique configurations due to differences in orbital energy.
- Applications in Chemistry: Fundamental for understanding periodic trends and anticipating chemical bonding interactions.
Tables, Diagrams, and Engaging Visual Aids
| Principal Energy Level | Sublevel | Number of Orbitals | Electron Capacity |
|---|---|---|---|
| 1 | s | 1 | 2 |
| 2 | s, p | 1, 3 | 2, 6 |
| 3 | s, p, d | 1, 3, 5 | 2, 6, 10 |
| 4 | s, p, d, f | 1, 3, 5, 7 | 2, 6, 10, 14 |
500K+ Students Use These Powerful Tools to Master Atomic Sublevels For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
423 flashcards
Flashcards on Atomic Sublevels
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards31 quizzes
Quizzes on Atomic Sublevels
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes14 questions
Exam questions on Atomic Sublevels
Boost your confidence with real exam questions.
Try Chemistry Questions3 exams created
Exam Builder on Atomic Sublevels
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Atomic Sublevels
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Atomic Sublevels you should explore
Discover More Revision Notes Related to Atomic Sublevels to Deepen Your Understanding and Improve Your Mastery
