Photo AI
Last Updated Sep 24, 2025
Schrödinger Equation Fundamentals Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Schrödinger Equation Fundamentals quickly and effectively.
323+ students studying
Schrödinger Equation Fundamentals
Overview
The Schrödinger Equation is pivotal for comprehending particle behaviour at the atomic scale. It can be regarded as a framework for predicting particle behaviour in quantum mechanics:
- Equation:
- Hamiltonian (): Denotes total energy.
- Wavefunction (): Describes the quantum state.
- Energy (): Indicates quantised energy levels.
Key Terms:
- Hamiltonian (): Operator expressing total energy.
- Wavefunction (): Represents the quantum state.
- Energy (): Represents quantised energy levels.
Historical Context and Key Limitations of the Bohr Model
Overview of the Bohr Model
- Bohr Model: Initially utilised for explaining hydrogen spectra.
- Applied fixed electron orbits akin to planets orbiting the sun.
Key Limitations of the Bohr Model
- Single-electron focus: Insufficient for multi-electron atoms.
- Inability to account for complex atomic spectra such as the Zeeman effect.
Key Limitations
- Single-electron focus
- Inability to elucidate electron interactions in complex atoms
- No acknowledgment of spectral phenomena like the Zeeman effect
Foundational Quantum Concepts
Wave-Particle Duality
- Displays both wave and particle characteristics. Evidenced in the double-slit experiment.
Probabilistic Interpretations
- Shift from deterministic to probabilistic models.
Quantisation Principles
- Electrons exist at distinct energy levels.
- Photon emissions occur during electron transitions between these levels.
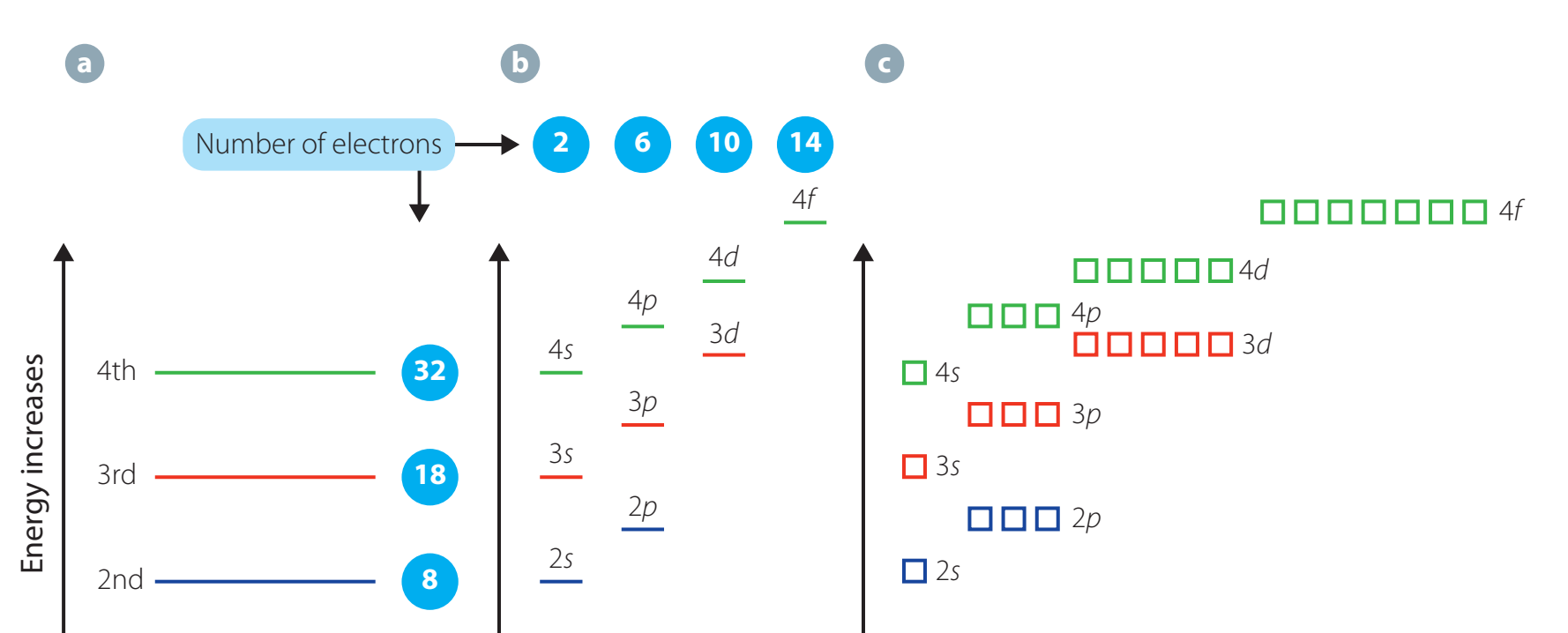
Quantum Numbers and Electron Configurations
Introduction to Quantum Numbers
Quantum numbers elucidate electron arrangements and their chemical implications:
- Principal Quantum Number (n): Corresponds to energy level and distance from the nucleus.
- Azimuthal Quantum Number (l): Defines orbital shape.
- Magnetic Quantum Number (m): Describes orbital orientation.
- Spin Quantum Number (m): Indicates electron spin direction.
Quantum numbers collectively form a 'unique electron address', essential for predicting atomic behaviour.
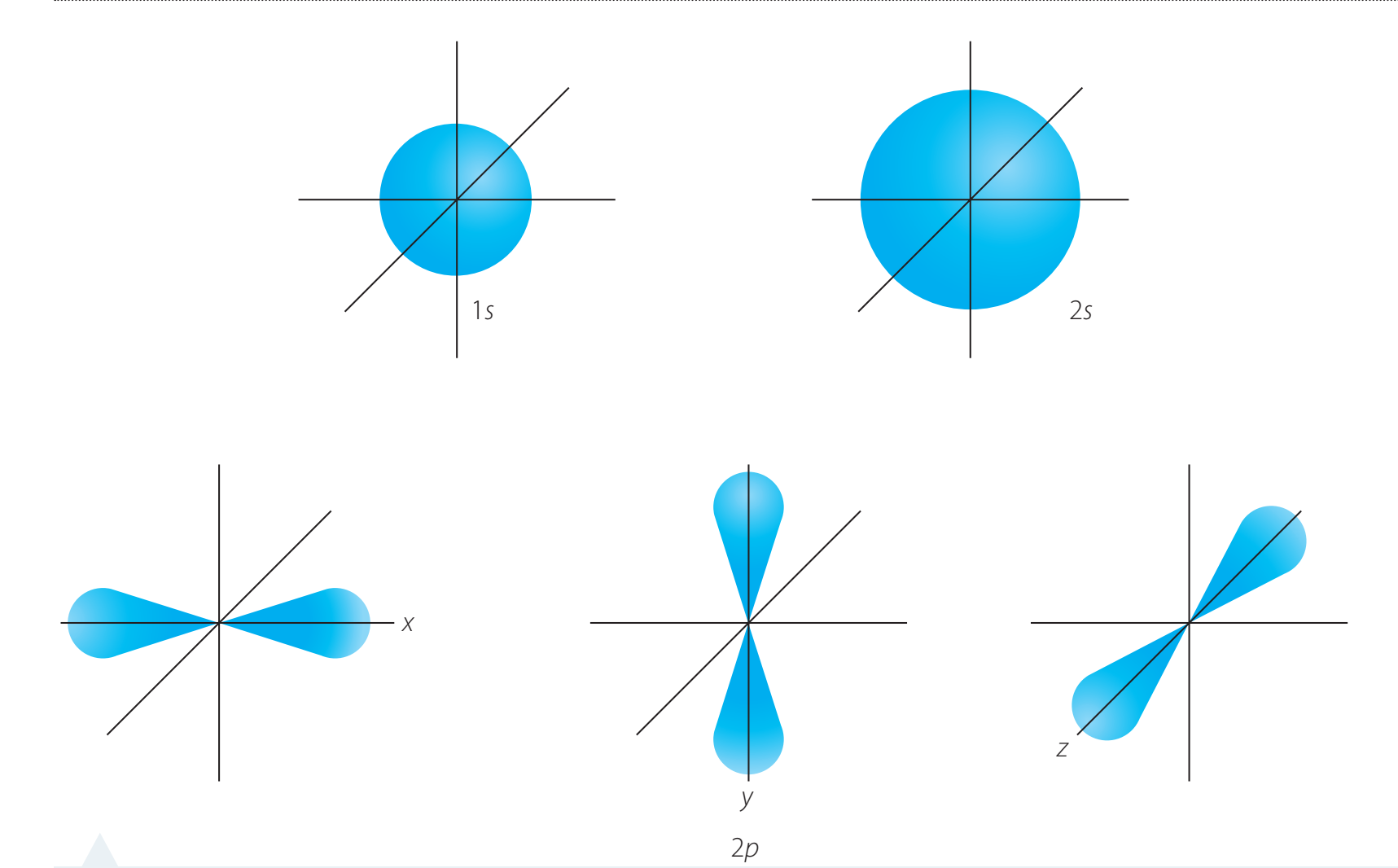
Orbital Shapes and Energies
- s Orbital: Spherical in shape
- p Orbitals: Dumbbell-shaped
- d Orbitals: Cloverleaf pattern
- f Orbitals: Complex with multiple lobes
Energy Quantisation
- Electrons reside in distinct energy levels. Predicts intricate energy configurations for multi-electron systems.
Schrödinger's model accurately forecasts energy levels for complex atoms.
The Schrödinger Equation's Role in Quantum Mechanics
Wave Functions and Probability Distributions
- Key Interpretations:
- Wavefunctions offer probabilistic descriptions of particle locations.
- Probability density is defined as .
From Orbits to Orbitals
- Shift to probability clouds.
- Quantum Numbers explain electron positions and energy states.
Applications: Spectroscopy and Atomic Spectra
Introduction to Spectral Techniques
- Purpose: Validate quantum models through light frequency emissions.
- Bohr Model Comparison:
- Successfully explained hydrogen's spectrum.
- Constrained in addressing complex atomic structures.
Spectral Complexities and Schrödinger's Advantages
- More massive elements' complex spectra necessitate Schrödinger's model.
- Employs electron probability clouds for enhanced prediction accuracy.

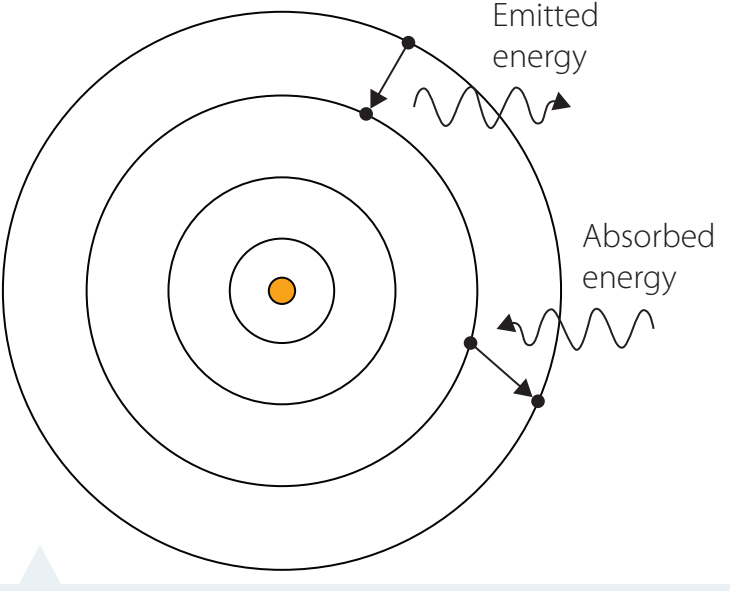
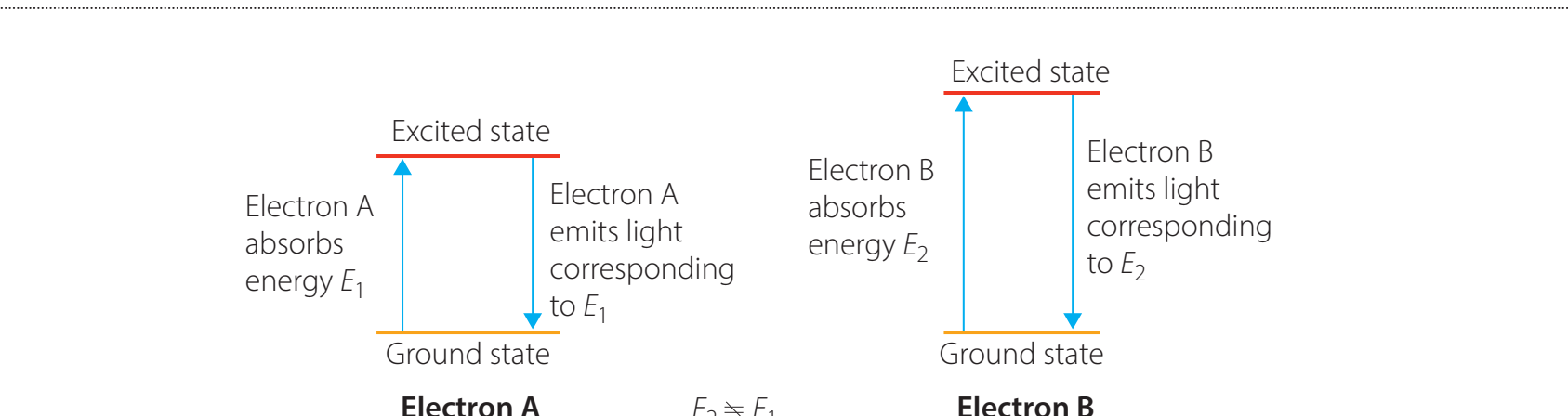
Visualising Spectral Transitions
-
Electron Transitions:
-
Produce spectral lines by emitting or absorbing photons.
-
Exam Tip: Grasping electron transitions and spectral lines is vital for exams.
Practical Techniques: Flame Tests
Introduction to Flame Tests
Flame tests identify metals via flame colour changes due to electron transitions.

Safety and Procedure
- Dissolve samples and utilise a Bunsen burner.
- Observe and document flame colours.
Always comply with safety procedures.
Observations and Spectroscope Use
- Maintain notebooks for observations.
- Employ a spectroscope for precision measurements.
Questions and Solutions
-
Question: Describe electron transitions responsible for flame colours. Solution: Flame colours result from electrons transitioning between energy levels. When electrons return to lower energy states, they emit photons with specific wavelengths corresponding to particular colours. For example, sodium produces a yellow flame due to electrons transitioning from the 3p to 3s orbital.
-
Question: Relate observations to Schrödinger's framework. Solution: Observations of flame colours directly validate Schrödinger's equation by demonstrating quantised energy transitions. The specific wavelengths emitted match the energy differences predicted by solving the Schrödinger equation for different elements.
-
Question: Predict flame colours for various ions based on transition data. Solution: Using transition energy data: Lithium (red, ~670 nm), Potassium (lilac, ~770 nm), Calcium (brick red, ~620 nm), Copper(II) (blue-green, ~450-530 nm), Barium (pale green, ~550 nm).
-
Question: Contrast results with theoretical models to assess spectral precision. Solution: Experimental flame test results should closely match theoretical wavelengths calculated from the Schrödinger equation. Any deviation might indicate experimental error or the influence of additional factors like impurities or competing transitions. For professional analysis, percentage error can be calculated as: .
This detailed comprehension of the Schrödinger Equation and its intrinsic quantum aspects lays a robust groundwork for advancing into atomic theory and chemistry.
500K+ Students Use These Powerful Tools to Master Schrödinger Equation Fundamentals For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
423 flashcards
Flashcards on Schrödinger Equation Fundamentals
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards31 quizzes
Quizzes on Schrödinger Equation Fundamentals
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes14 questions
Exam questions on Schrödinger Equation Fundamentals
Boost your confidence with real exam questions.
Try Chemistry Questions3 exams created
Exam Builder on Schrödinger Equation Fundamentals
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Schrödinger Equation Fundamentals
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Schrödinger Equation Fundamentals you should explore
Discover More Revision Notes Related to Schrödinger Equation Fundamentals to Deepen Your Understanding and Improve Your Mastery
