Photo AI
Last Updated Sep 24, 2025
Unstable Isotopes Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Unstable Isotopes quickly and effectively.
235+ students studying
Unstable Isotopes
Basic Definitions
Isotopes: Atoms of a single element that have the same number of protons but vary in their number of neutrons.
- They reside at the same position on the periodic table due to their identical atomic numbers.
- This concept is significant in disciplines such as nuclear chemistry and environmental science.
Radioactive decay is a process where unstable nuclei lose energy by emitting radiation.
Characteristics of Isotopes
- Isotopes share positions on the periodic table because of the identical number of protons.
- Their chemical behaviour is largely the same due to sharing the same electron configuration.
Isotope Stability
Stable Isotopes
- Stable Isotopes: These do not undergo radioactive decay.
- They are applied in medicine and environmental monitoring because of their enduring stability.
Unstable Isotopes (Radioisotopes)
- Unstable Isotopes: These emit radiation as they decay.
- Example: Carbon-14 decays into stable Nitrogen-14.
It is essential to understand how certain conditions influence the change from radioisotope to stable isotope, which is pivotal in fields like medical imaging and environmental monitoring.
Neutron-to-Proton Ratio and Isotope Stability
- The Neutron-to-Proton (n/p) Ratio is crucial for assessing the stability of an isotope.
- The Zone of Stability denotes naturally stable isotopes based on their n/p ratios.
Introduction to Radioactivity
- Radioactivity entails the release of energy and particles as atoms transition from an unstable state to a stable state.
- Instability often results from an imbalance between protons and neutrons.
Half-Life:
- The period required for half of the atoms in a radioactive sample to decay.
- It is essential in areas such as archaeology and medicine.
Half-Life Example: Radioisotopes reduce by half during each half-life period, similar to sand passing through an hourglass.
Decay Processes in Unstable Isotopes
Alpha, Beta, and Gamma Decay
Alpha Decay
- Expulsion of an alpha particle (2 protons, 2 neutrons).
- Example: Uranium-238 decays to Thorium-234.
Beta Decay
- Involves the conversion of a neutron into a proton, releasing a beta particle.
- Example: Carbon-14 converts to Nitrogen-14.
Gamma Decay
- Emission of gamma rays, which typically occur after alpha or beta decay.
- Utilised in medical imaging due to non-ionisation of mass.
Types of Radiation
Alpha Radiation
- Composed of 2 protons and 2 neutrons.
- Possesses a large mass and a positive charge.
- Exhibits low penetration power; can be stopped by paper.
Beta Radiation
- Comprises high-speed electrons or positrons.
- Has medium penetration power and is suitable for medical diagnostics like PET scans.
Gamma Radiation
- Composed of high-energy photons without mass or charge.
- Exhibits high penetration power, requiring dense materials for shielding; widely applied in cancer treatment.
Safety Considerations and Shielding
- Protective Measure: Implement appropriate shielding and minimise exposure time.
ALARA Principles:
- As Low As Reasonably Achievable.
- Emphasise suitable shielding and limit exposure time.
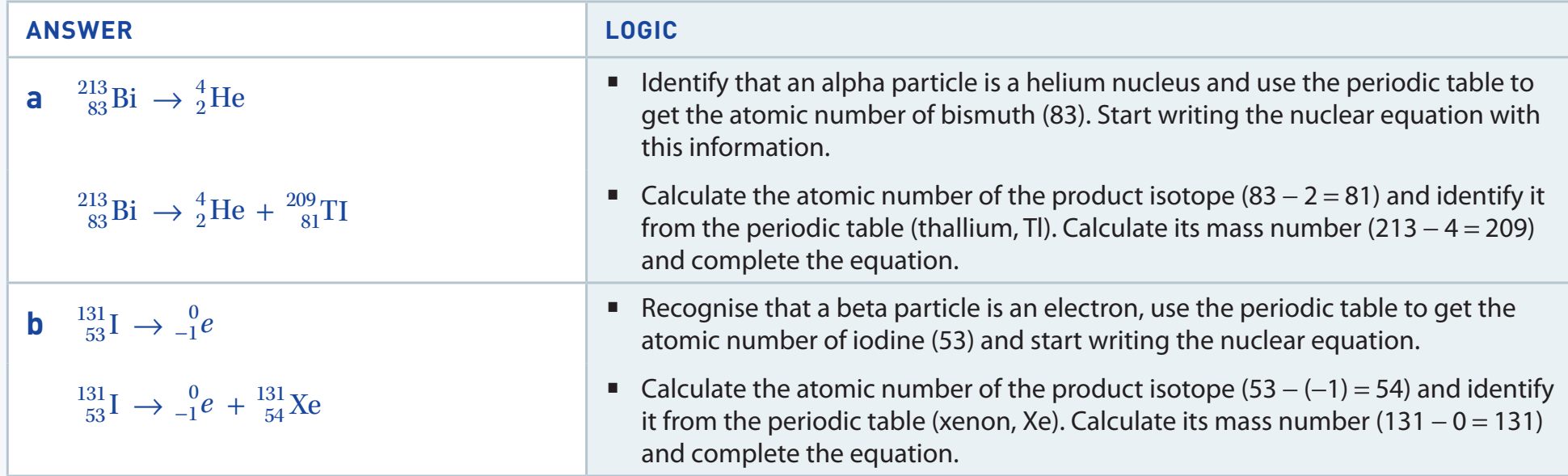
Balanced Nuclear Reactions
Studying nuclear reactions reveals fundamental differences from chemical reactions, concentrating on transformations within the atomic nucleus.
Types of Nuclear Reactions
Alpha, Beta, and Gamma Processes
- Adheres to principles of conservation of mass and atomic numbers in reactions.
- Accurate calculation of daughter nuclei post-transitions is crucial.
Diagrammatic Explanations
- Alpha Decay: Uranium-238 transitions to Thorium-234.
- Beta Decay: Carbon-14 transitions to Nitrogen-14.
- Offers comprehensive visuals detailing each decay process.
Illustrative Examples
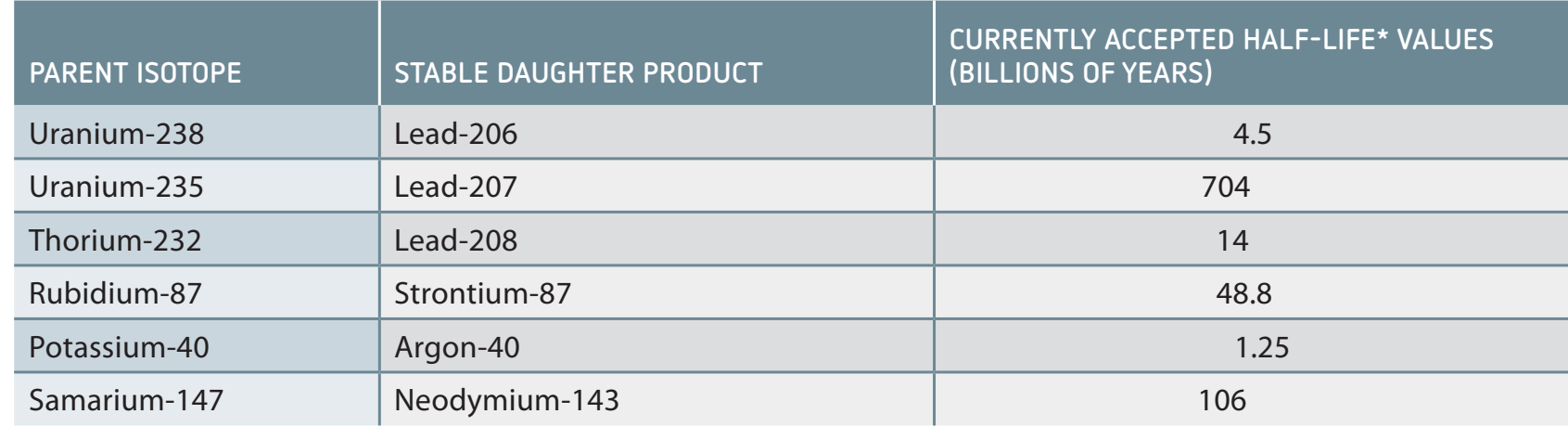
- Decay Chain of Uranium-238: Provides insights into transformation into stable lead through multiple decay phases.
- Geiger Counter: Essential device for monitoring nuclear activity around isotopes.

500K+ Students Use These Powerful Tools to Master Unstable Isotopes For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
423 flashcards
Flashcards on Unstable Isotopes
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards31 quizzes
Quizzes on Unstable Isotopes
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes14 questions
Exam questions on Unstable Isotopes
Boost your confidence with real exam questions.
Try Chemistry Questions3 exams created
Exam Builder on Unstable Isotopes
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Unstable Isotopes
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Unstable Isotopes you should explore
Discover More Revision Notes Related to Unstable Isotopes to Deepen Your Understanding and Improve Your Mastery
