Photo AI
Last Updated Sep 24, 2025
Chemical Synthesis and Yield Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Chemical Synthesis and Yield quickly and effectively.
203+ students studying
Chemical Synthesis and Yield
Overview of Chemical Synthesis
- Chemical Synthesis: The process of integrating elements or compounds to form a specific product.
- Significance: Plays a crucial role in developing products vital for daily life and various industries.
Role in Industrial Applications
- Pharmaceuticals: Essential for producing active medicinal ingredients.
- Example: Aspirin, a commonly used drug, is synthesised through the acetylation of salicylic acid.
- Agriculture: Fertilisers and pesticides are synthesised to enhance food production.
- Example: Ammonia, a fundamental ingredient in fertilisers, is produced via the Haber process.
- Consumer Goods: Utilised in products like plastics.
- Example: Polymer synthesis is employed for various consumer goods.
- Societal Implications: Synthesis stimulates economic growth and improves the quality of life by providing essential goods.
- Example: Increased food production enhances food security.
Inquiry Question Introduction
- Syllabus Focus: "What are the implications for society of chemical synthesis and design?"
- Societal Implications: Advances society by providing solutions to health and productivity challenges.
- Environmental Implications: Ensures development is balanced with resource conservation.
- Relevance: Essential in addressing societal challenges through innovative approaches.
Importance of Efficient Synthesis
- Efficient Synthesis: Processes that minimise waste and maximise productivity.
- Waste Reduction: Critical for diminishing the environmental footprint by generating less chemical waste.
- Environmental & Economic Impact: Efficient synthesis results in lower carbon emissions and cost reductions.
Efficient chemical synthesis is imperative for reducing waste and enhancing sustainability, which is crucial in contemporary industrial dynamics.
Technological Advancements
- Specific Advancements: Innovations in green chemistry, including renewable energy resources.
- Example: Catalytic converters transform harmful emissions into less harmful substances.
- Impact: Enhances efficiency, reduces energy consumption, and promotes sustainability.
- Further Examples: In pharmaceuticals, synthesis of complex molecules for viruses; in agriculture, biopesticide synthesis boosts crop yields.
Understanding Reaction Yield
- Reaction Yield: Compares the amount of product formed in a reaction to the maximum possible amount. It is a key metric for efficiency and productivity, essential for reducing costs and minimising environmental impact.
Reaction Yield: Indicates the efficiency and productivity of a chemical process.
Definition and Significance
- Reaction Yield: An efficiency measure crucial for optimising processes and minimising costs and waste.
- Plays a vital role in reducing environmental impact.
Key Concepts
-
Theoretical Yield:
- Definition: The maximum product amount predicted from balanced chemical equations.
- Example: Starting with 1 mol of reactant A ideally produces 1 mol of product B.
-
Actual Yield:
- Definition: The real amount of product obtained from an experiment.
- Example: If only 0.9 mol of B is obtained from the expected 1 mol.
-
Percentage Yield:
- Definition: An efficiency measure comparing actual yield to theoretical yield.
- Formula:
- Important for evaluating industrial efficiency.
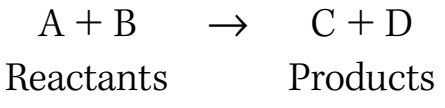
Factors Affecting Reaction Yield
-
Purity of Reactants:
- Impurities may cause side reactions, reducing yield.
- High purity is crucial to prevent yield reduction.
infoNoteEnsure high purity to avert side reactions.
-
Reaction Conditions:
Temperature and Pressure:
- Major factors influencing reaction rates and equilibria.
- Example: In the Haber Process, low temperature increases yield but reduces rate; high pressure favours ammonia production.
Catalyst Use:
- Accelerates reaction rates without altering equilibrium.
- Common catalysts: Iron in the Haber Process accelerates ammonia synthesis; Platinum in cars decreases emissions.
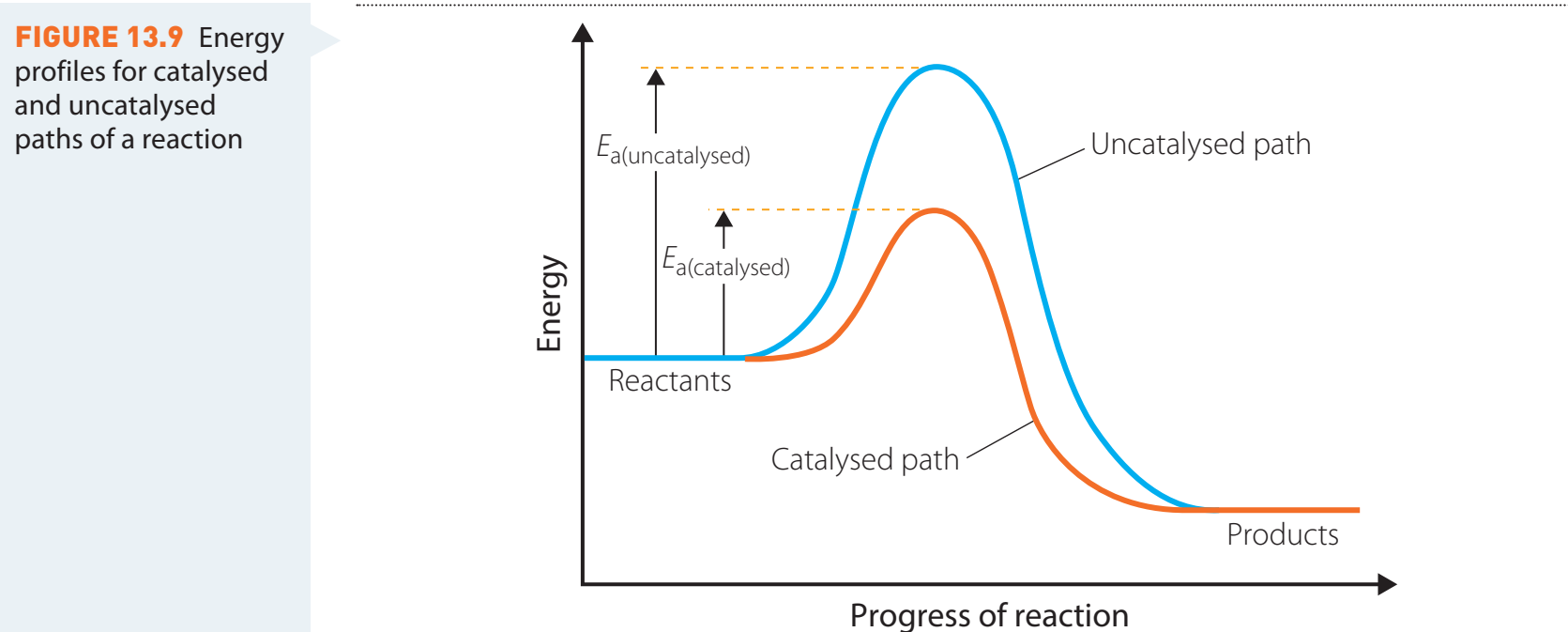
Equilibrium Considerations
-
Le Chatelier's Principle:
- Utilised to predict yield changes when altering conditions.
- Exam Tip: Apply this principle to predict equilibrium shifts during exams.
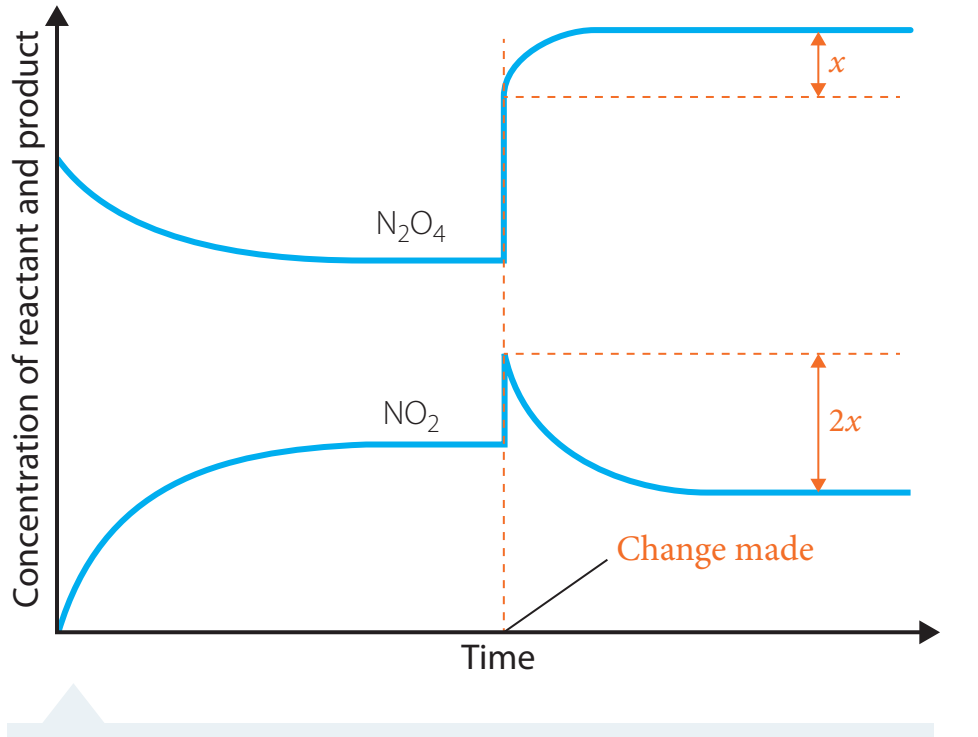
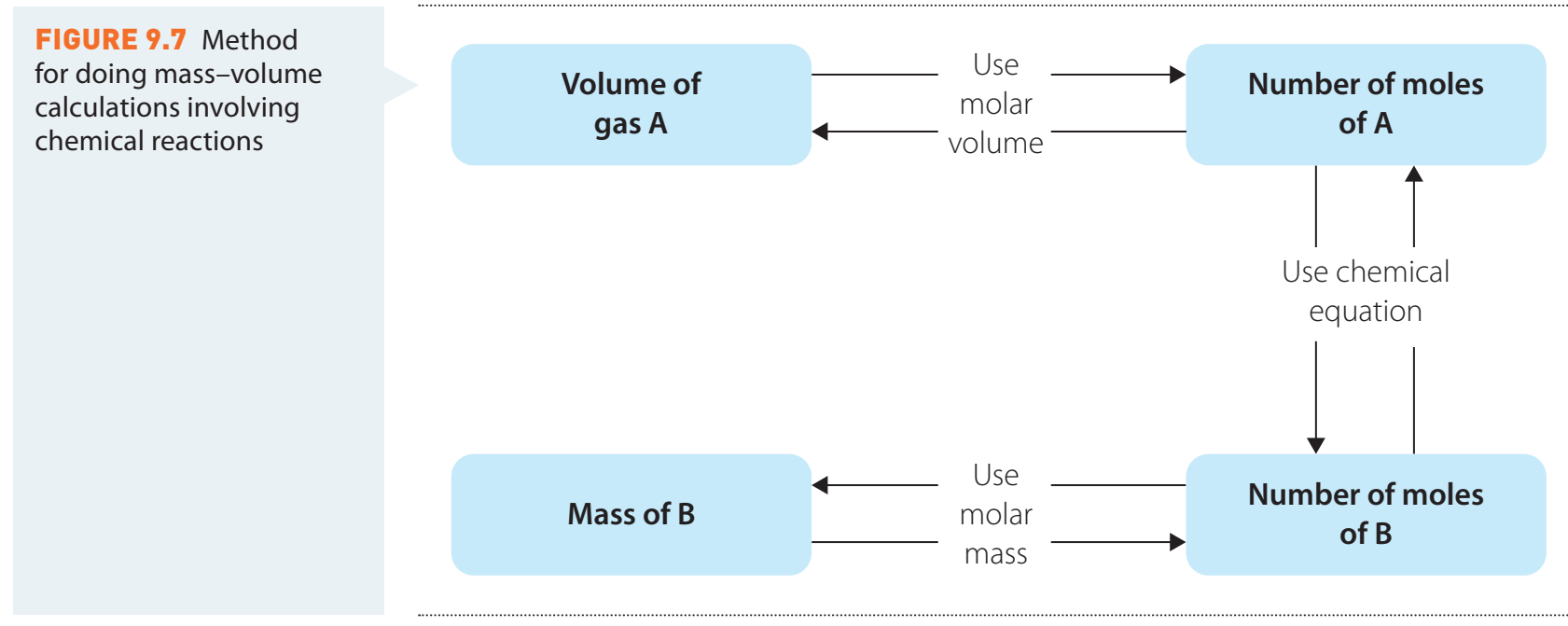
Prediction and Calculation of Yield
- Limiting Reagents: Determines the maximal amount of product by being consumed first.
- Theoretical Yield Calculation involves stoichiometry to predict maximum expected product based on balanced equations.
- Percentage Yield:
- Accurate stoichiometry is necessary for determining correct reactant and product amounts.
Worked Example
- Scenario: 5g of reactant A should yield 10g of product B, but only 8g is obtained.
- Calculation:
- Theoretical Yield = 10g
- Actual Yield = 8g
- Percentage Yield = (8/10) × 100% = 80%
- Explanation: The actual yield is 80% of what was theoretically possible. This is a common scenario in real chemical processes where various factors like incomplete reactions, side reactions, or loss during product isolation can reduce efficiency.
A. Importance of Yield in Industrial Chemical Synthesis
- Yield: The amount of product obtained compared to the maximum possible. Measures the reaction's effectiveness.
- Pharmaceuticals: Ensures consistent quality and profitability.
- Plastics: Reduces energy consumption and costs, lowers environmental impact.
- Agrochemicals: Maximises effectiveness.
Yield determines productivity and cost-efficiency across various sectors.
B. Economic and Environmental Implications
-
Economic Benefits:
- High yields save material costs, boosting profitability.
infoNoteMaterial Costs: Reducing these costs can lead to competitive pricing advantages.
-
Environmental Benefits:
- High yields minimise waste and energy usage.
infoNoteGreen Chemistry aims to curtail hazardous waste and lessen environmental impact.
C. Conclusion on Yield Optimization
- Yield optimisation is vital for aligning financial success with environmental objectives.
- Consider the importance of reaction yield in enhancing efficiency in chemical production. It is crucial for real-world applications.
500K+ Students Use These Powerful Tools to Master Chemical Synthesis and Yield For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
182 flashcards
Flashcards on Chemical Synthesis and Yield
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards14 quizzes
Quizzes on Chemical Synthesis and Yield
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes34 questions
Exam questions on Chemical Synthesis and Yield
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Chemical Synthesis and Yield
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Chemical Synthesis and Yield
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Chemical Synthesis and Yield you should explore
Discover More Revision Notes Related to Chemical Synthesis and Yield to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Chemical Synthesis and Design
Chemical Synthesis and Design
251+ studying
184KViews