Photo AI
Last Updated Sep 24, 2025
Energy Profiles Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Energy Profiles quickly and effectively.
223+ students studying
Energy Profiles
Energy profiles: Graphical tools illustrating energy transformations during chemical reactions. These profiles elucidate the following:
- Activation Energy: The essential minimum energy required for reactants to convert into products, functioning as an energy threshold that must be crossed for reactions to commence.
- Enthalpy Change (ΔH): The total energy difference between reactants and products.
- Educational Purpose: Aids in comprehending intricate chemical processes and reaction mechanics.
- Activation Energy: Energy necessary to initiate a reaction
- Enthalpy Change: Energy gap between reactants and products
- Educational Tool: Assists in grasping reaction dynamics
Basic Components of Energy Profiles
- Reactants and Products: Indicate the initial and concluding energy states.
- Transition State: The apex of the energy profile representing the highest energy level, referred to as the activated complex.
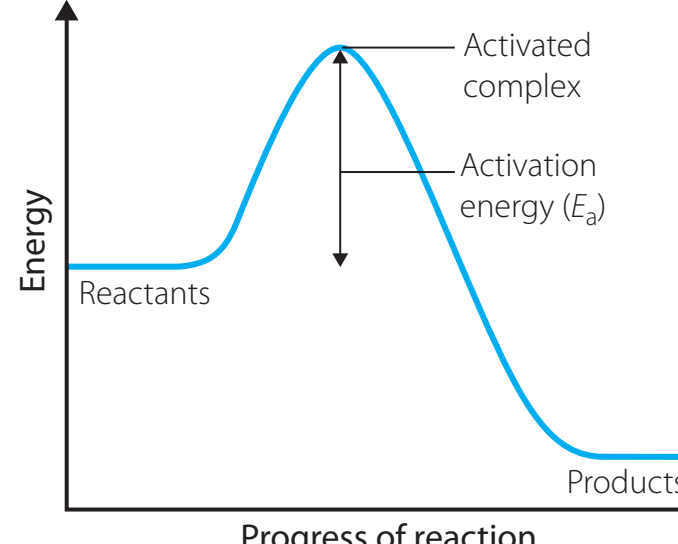
Exothermic vs. Endothermic Reactions
- Exothermic Reactions:
- Example: Combustion of Methane, characterised by energy release, depicted as a decline in energy.
- Endothermic Reactions:
- Example: Photosynthesis, marked by energy absorption, depicted as an increase in energy.
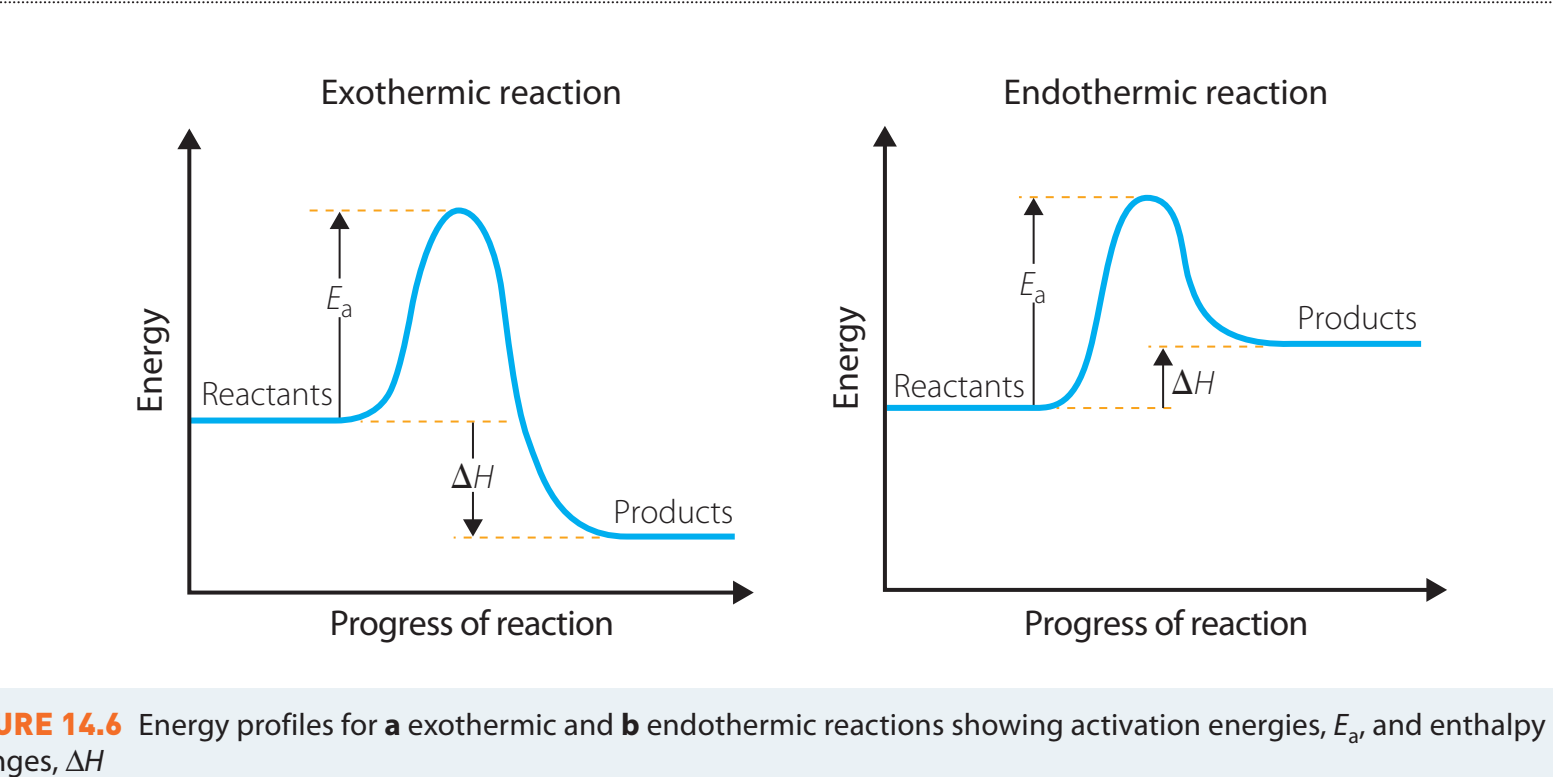
Activation Energy and Enthalpy Change
Important Concepts
-
Activation Energy (Ea):
- Definition: The minimum energy necessary for reactants to be converted into products, representing the energy barrier that must be surpassed for a reaction to proceed.
- Influence: Adjustable by temperature and catalysts, which can enhance or impede reaction rates.
-
Enthalpy Change (ΔH):
- Definition: The energy released or absorbed in a reaction, calculated as the difference in energy between products and reactants.
- Endothermic Reactions: ΔH is positive—indicating energy absorption.
- Exothermic Reactions: ΔH is negative—indicating energy release.

Activation energy should not be confused with the total reaction energy. It is distinct from the enthalpy change.
Catalyst Function and Energy Profile Changes
- Catalysts: Agents that increase the rate of reaction without being consumed in the process.
- Diagram Interpretation: Catalysts reduce the activation energy level, as shown by a lower peak on the energy profile.
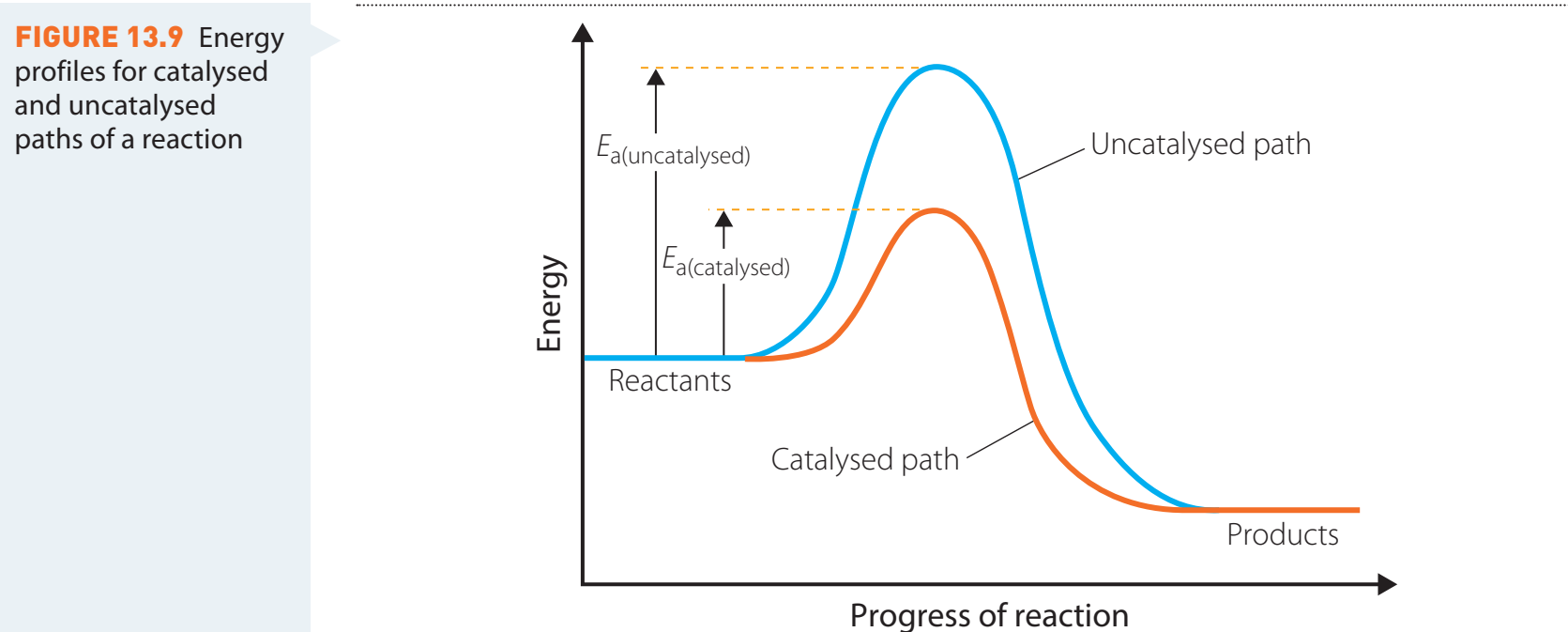
Types of Catalysts
- Homogeneous: Exists in the same phase as reactants (e.g., sulphuric acid in esterification).
- Heterogeneous: Exists in a different phase (e.g., iron in the Haber process).
- Biological: Enzymes such as catalase, which decomposes hydrogen peroxide.
Relationship Between Energy Profiles and Reaction Mechanisms
- Reaction Mechanism: The sequence of elementary steps that define complex reactions.
- Intermediate: Temporary species formed during the reaction steps.
- Transition State: Short-lived, high-energy states during the reaction.
- Rate-Determining Step: The slowest step that influences the overall reaction rate.

Transition states are fleeting high-energy configurations that cannot be isolated.
Influence of Catalysts
- Catalysts Effect: Introduce alternative pathways with lower energy requirements, thereby simplifying reaction mechanisms.

Catalysts effectively lower energy barriers by creating more efficient reaction pathways.
Worked Examples
Example 1: Calculating Enthalpy Change
For an exothermic reaction:
- Reactants energy: 50 kJ/mol
- Products energy: 30 kJ/mol
- Enthalpy change: kJ/mol
- This negative value confirms the reaction is exothermic (releases energy)
Example 2: Identifying Reaction Type
For a reaction with:
- Reactants energy: 60 kJ/mol
- Products energy: 80 kJ/mol
- Enthalpy change: kJ/mol
- This positive value indicates an endothermic reaction (absorbs energy)
500K+ Students Use These Powerful Tools to Master Energy Profiles For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
198 flashcards
Flashcards on Energy Profiles
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards19 quizzes
Quizzes on Energy Profiles
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes13 questions
Exam questions on Energy Profiles
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Energy Profiles
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Energy Profiles
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Energy Profiles you should explore
Discover More Revision Notes Related to Energy Profiles to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Energy Changes in Chemical Reactions
Energy Changes in Chemistry
262+ studying
190KViews96%
114 rated
Energy Changes in Chemical Reactions
Enthalpy and Enthalpy Change
483+ studying
197KViews96%
114 rated
Energy Changes in Chemical Reactions
Heat and Chemical Reactions
315+ studying
186KViews