Photo AI
Last Updated Sep 24, 2025
Unsaturated Hydrocarbon Reactions Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Unsaturated Hydrocarbon Reactions quickly and effectively.
287+ students studying
Unsaturated Hydrocarbon Reactions
This document details the reactions involving unsaturated hydrocarbons, with a particular emphasis on alkenes and alkynes. A thorough understanding of these reactions is vital for their application in industrial and laboratory contexts.
Definitions and Distinction
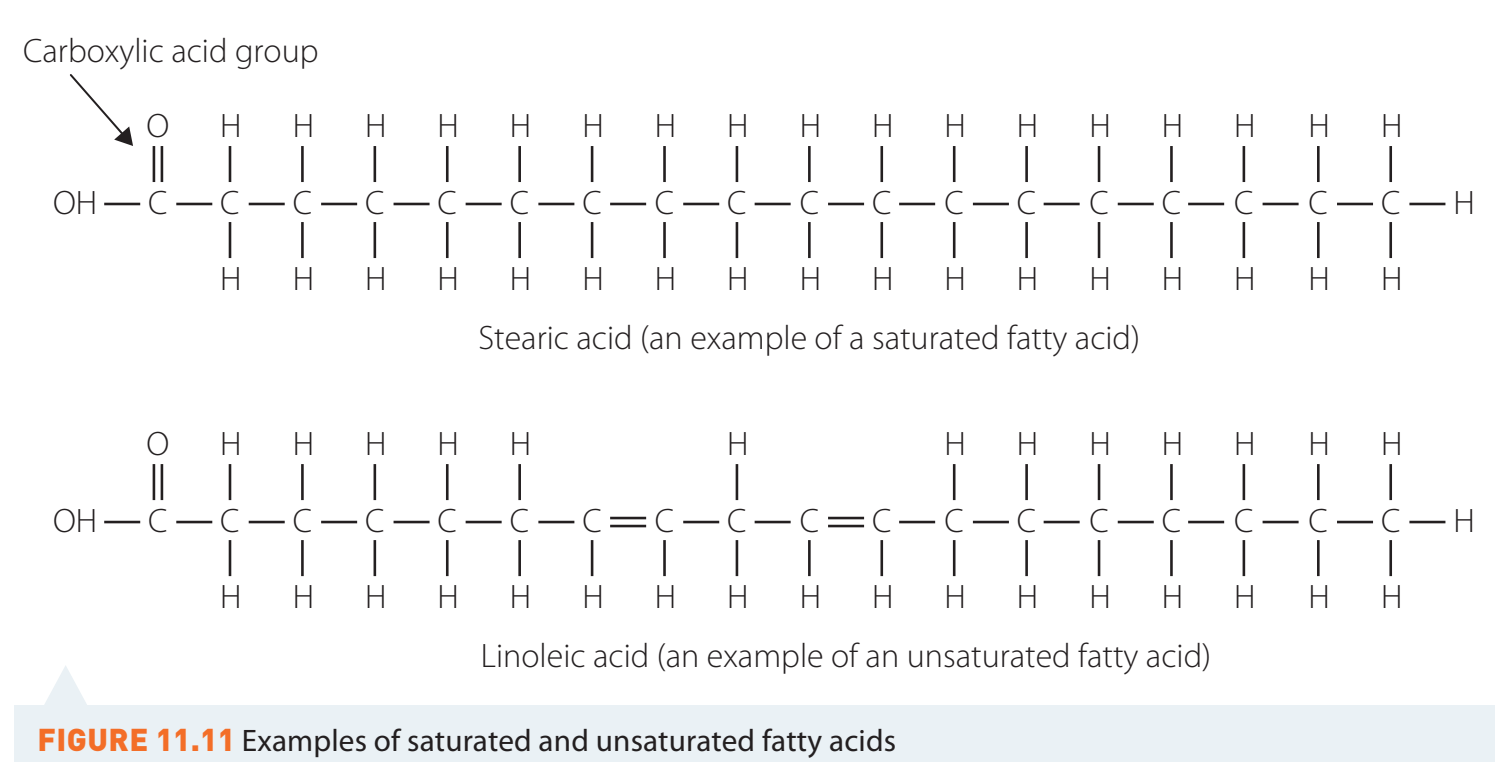
- Unsaturated Hydrocarbons: These are compounds characterised by the presence of double (C=C) or triple (C≡C) bonds, such as alkenes and alkynes. Such bonds confer higher reactivity compared to saturated hydrocarbons.
- Example: Ethylene (CH) is an alkene, while acetylene (CH) is an alkyne.
- Influence on Physical Properties: The presence of unsaturation influences boiling and melting points.
- Example: Due to its double bond, ethylene has a lower boiling point than ethane.
Alkenes and alkynes display unique reactivity and physical characteristics due to their multiple bonds.
Structures of Alkenes and Alkynes
- General Formulas:
- Alkenes: CH.
- Alkynes: CH.
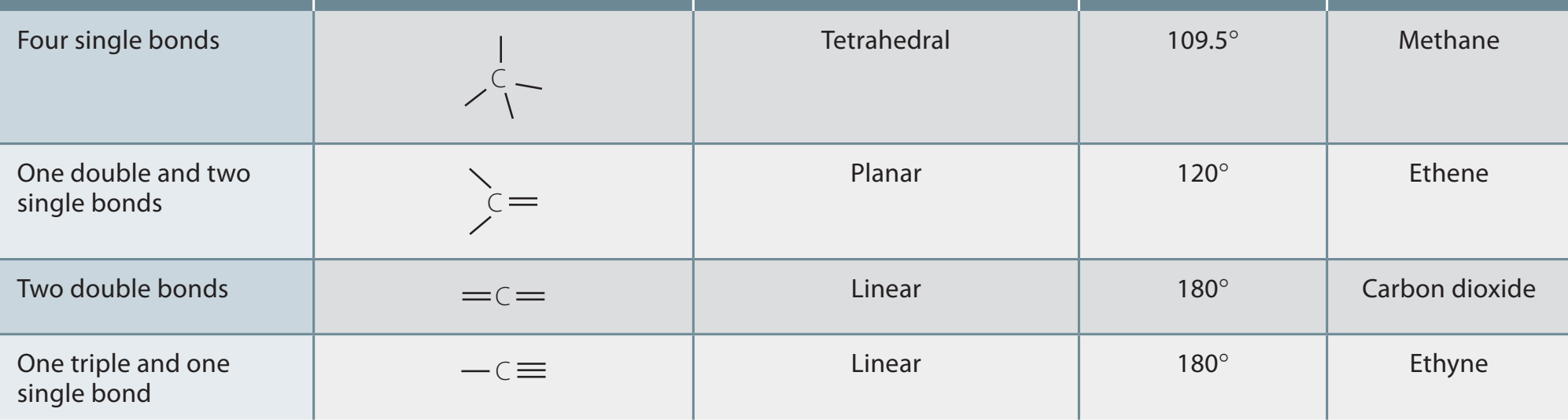
- Bonding and Geometry:
- The double bonds in alkenes impart a planar structure, influencing polarity and reactivity.
- Alkynes, with their triple bonds, have a linear geometry, impacting reactivity and bond angles.
Understanding the geometry of these bonds aids in predicting their interactions during reactions.
Pi Bonds and Reactivity
- Pi Bonds:
- These arise from the lateral overlap of p orbitals, forming reactive electron clouds.
- Pi bonds are more reactive than sigma bonds, enabling addition reactions.
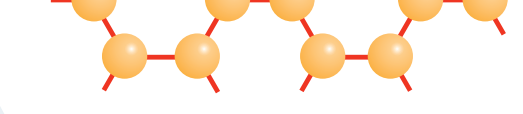
Addition Reactions Overview
- Addition Reactions: These reactions involve the addition of atoms or groups to the multiple bonds in alkenes or alkynes without any loss of atoms.
Addition Reaction: Characterised by the incorporation of atoms into alkenes or alkynes, with pi bonds offering a reactive pathway.
Detailed Mechanism of Addition Reactions
- Step-by-Step Breakdown:
- Step 1: Interaction between pi bonds and another molecule.
- Step 2: Formation of intermediates, which may involve charged species.
- Step 3: The formation of stable sigma bonds concludes the reaction.
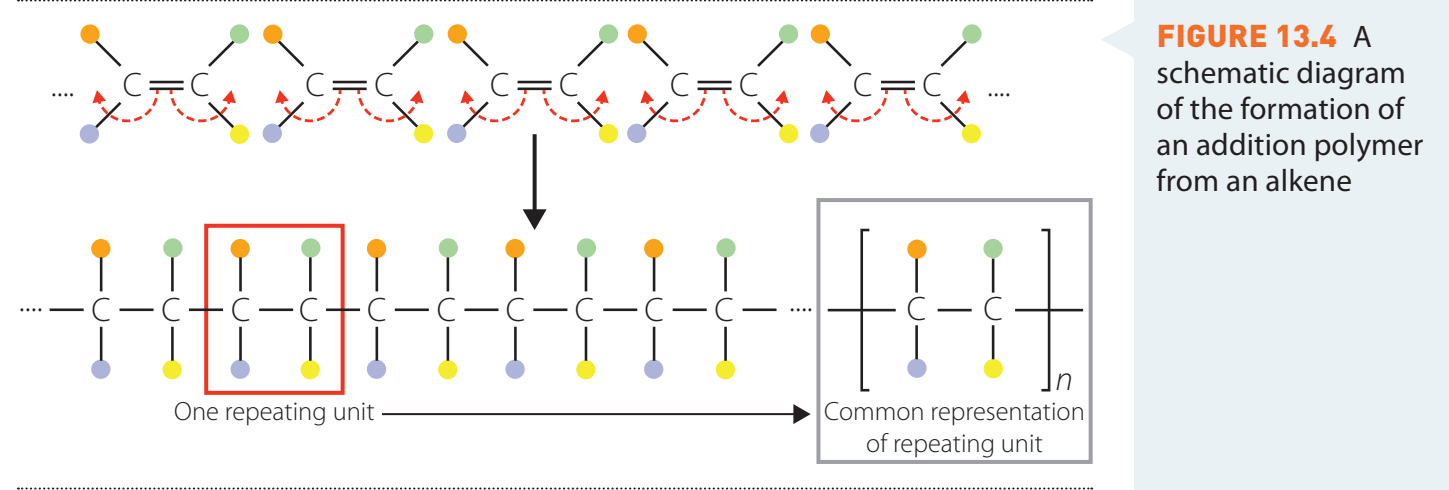
Hydrogenation Reactions
- Hydrogenation: This process involves adding hydrogen to unsaturated hydrocarbons, converting them into saturated hydrocarbons.
Hydrogenation: The addition of hydrogen to unsaturated hydrocarbons to achieve saturation.
Mechanism of Hydrogenation
- Step 1: Hydrogen adsorption onto a metal catalyst.
- Step 2: Cleavage of H into individual atoms.
- Step 3: Transition states form, converting pi bonds into sigma bonds.
The selection of a metal catalyst is vital in determining the efficiency of hydrogenation.
Halogenation Reactions
- Halogenation: A reaction where halogens are added to unsaturated compounds.
- Common halogens used include chlorine (Cl) and bromine (Br).
- Electrophilic Addition:
- The halogen approaches the electron-rich pi bond.
- Formation of a halonium ion occurs.
- Halogen atoms add across the bond.

Hydrogen Halide Reactions
- Hydrogen Halides (HX): Compounds like HCl, HBr, and HI, consisting of hydrogen and halogens.
- They react with unsaturated hydrocarbons via electrophilic addition.
Role of Markovnikov's Rule
- Markovnikov's Rule: The halogen attaches to the more substituted carbon in the molecule.
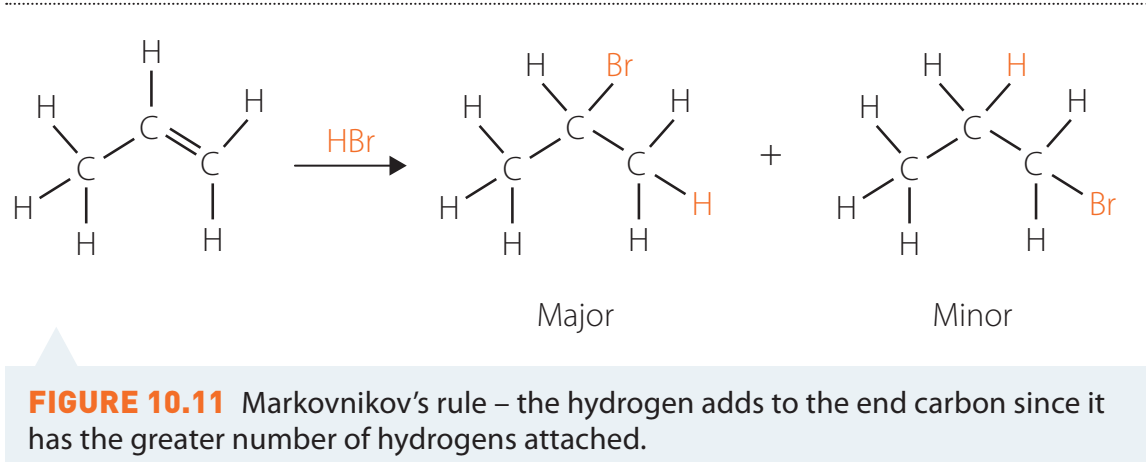
Hydration Reactions
- Hydration: Involves the addition of water to unsaturated hydrocarbons using an acidic catalyst.
Definition of Hydration: Water is added to hydrocarbons in the presence of an acidic catalyst.
Detailed Mechanism of Hydration Reaction
- Initiation: An acid catalyst donates a proton, starting the reaction process.
- Carbocation Stabilisation: Essential for the progression of the reaction.
- Product Formation: A nucleophilic attack results in the formation of an alcohol.
Model Construction and Equation Writing
Building Molecular Models
- Tools: Utilise ball-and-stick kits to visualise molecular structures.
- Exercise: Construct models of ethylene (CH) and acetylene (CH).
Writing Reaction Equations
- Example:
- Hydrogenation of ethylene:
Stereochemistry and Geometry
- Visualising Stereoisomers: Crucial for real-world applications.
Worked Examples
Example 1: Hydrogenation of Propene
Propene undergoes hydrogenation to form propane:
Solution:
- The double bond in propene (C=C) reacts with H₂ in the presence of a catalyst
- Hydrogen atoms add across the double bond
- The product is propane, a saturated hydrocarbon
Example 2: Hydration of Ethene
Ethene reacts with water in the presence of an acid catalyst to form ethanol:
Solution:
- The acid catalyst protonates the double bond, forming a carbocation
- Water attacks the carbocation to form an oxonium ion
- Deprotonation yields ethanol as the final product
Example 3: Halogenation of Butene
When 2-butene reacts with bromine, 2,3-dibromobutane is formed:
Solution:
- Bromine forms a cyclic bromonium ion intermediate with the alkene
- The bromide ion attacks from the opposite side (anti addition)
- This results in the trans configuration in the final product
Focus on understanding reaction mechanisms rather than memorising individual reactions for better conceptual clarity.
500K+ Students Use These Powerful Tools to Master Unsaturated Hydrocarbon Reactions For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
107 flashcards
Flashcards on Unsaturated Hydrocarbon Reactions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards14 quizzes
Quizzes on Unsaturated Hydrocarbon Reactions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes15 questions
Exam questions on Unsaturated Hydrocarbon Reactions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Unsaturated Hydrocarbon Reactions
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Unsaturated Hydrocarbon Reactions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Unsaturated Hydrocarbon Reactions you should explore
Discover More Revision Notes Related to Unsaturated Hydrocarbon Reactions to Deepen Your Understanding and Improve Your Mastery
