Photo AI
Last Updated Sep 26, 2025
Naming Inorganic Compounds Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Naming Inorganic Compounds quickly and effectively.
489+ students studying
Naming Inorganic Compounds
Introduction
Understanding the components and structure of inorganic compounds is essential for predicting characteristics and proper nomenclature. This foundational knowledge supports scientific study and practical applications. Inorganic acids and bases are vital for accurate chemical communication and laboratory safety, as incorrect labelling can lead to hazardous situations.
Purpose of Nomenclature
- Nomenclature: A systematic method of naming chemicals.
- Ensures uniform communication in chemical sciences.
- Prevents ambiguity in substance identification.
- Facilitates global communication among scientists.
Example: Without a systematic nomenclature, chemicals might have different names across regions, leading to confusion. Imagine a scenario where the same compound is known by multiple names—scientists could find collaboration challenging.
IUPAC's Role
- IUPAC: International Union of Pure and Applied Chemistry.
- Establishes global norms for chemical naming.
- Enhances clarity in international scientific communication.
Example of Influence: Before IUPAC, names like methane varied worldwide. Today, methane is universally recognised, thanks to IUPAC's standardisation efforts.
Historical Context
-
Over time, chemical naming evolved from diverse methods to more structured approaches.
-
This timeline outlines significant milestones in IUPAC's contributions:

-
These advancements have established clarity in the chemistry domain, maintaining relevance across generations.
Basic Structure of Inorganic Compounds
-
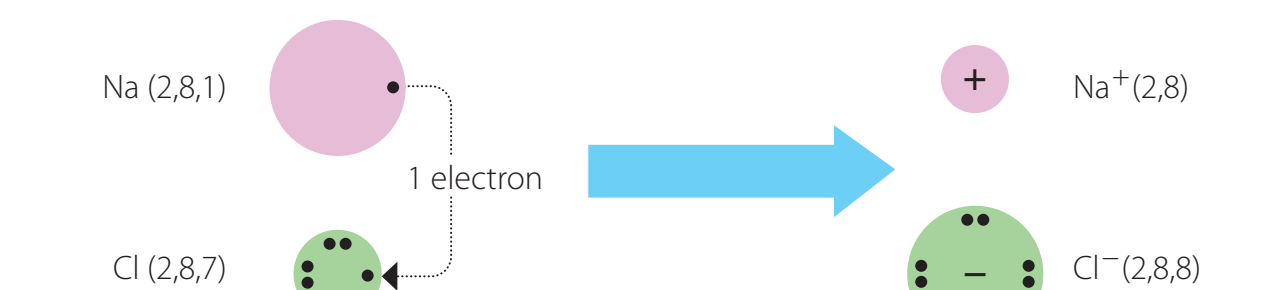
Inorganic Compounds: Comprise cations and anions forming ionic bonds.
-
Cations: Positively charged ions.
-
Anions: Negatively charged ions.
-
Sodium Chloride Example: Demonstrates ionic bonding, leading to stable structures.
Network Covalent Substances: Unique structures, such as silicon dioxide (SiO₂), possess distinctive properties due to extensive covalent bonds.
Influence of Molecular Structure on Naming
- Atomic Arrangement: Affects naming using IUPAC conventions.
- Structural Formulas: Essential for deriving correct names.
| Compound | Molecular Formula | Empirical Formula |
|---|---|---|
| Water | H₂O | H₂O |
| Glucose | C₆H₁₂O₆ | CH₂O |
Naming Inorganic Acids
Systematic Naming of Inorganic Acids
-
Binary Acids Naming
- Binary acids consist of hydrogen and a non-metal.
- Naming sequence:
- Use the prefix 'hydro-'
- Follow with the root of the non-metal
- Add the suffix '-ic'
- Conclude with 'acid'
Example: is hydrochloric acid.
infoNoteName binary acids by combining 'hydro-', the root of the non-metal, and '-ic', finishing with 'acid'.
-
Oxyacids Naming
- Oxyacids contain hydrogen, oxygen, and another element.
- '-ate' ions transform into '-ic' acids, and '-ite' ions transform into '-ous' acids.
Examples:
- : sulphuric acid
- : sulphurous acid
Oxyacids rely on suffix adjustments, converting '-ate' to '-ic' and '-ite' to '-ous', based on the polyatomic ion.
Naming Inorganic Bases
- Components of Inorganic Bases
- Mainly composed of metallic cations combined with hydroxide/oxide ions.
Structure: Metallic Cation + Hydroxide/Oxide ion (e.g., NaOH).
IUPAC Naming Rules for Inorganic Bases
- Naming Convention: Combine the name of the metal cation and hydroxide or oxide.
- E.g., NaOH is sodium hydroxide.
- E.g., K₂O is potassium oxide.
Oxidation States: Specify oxidation states using parentheses, such as iron(III) hydroxide.
Importance in Scientific Communication
- Systematic naming:
- Facilitates academic exchange globally.
- Ensures reproducibility and clarity in research.
- Essential for global data sharing, enhancing understanding across borders.
Common Errors and Solutions
- For Exams and Studies: Standard naming simplifies understanding in test settings and supports learning in international contexts.
Incorrect chemical naming can result in significant research mishaps. Misidentifying hydrochloric (HCl) as hypochlorous (HClO) acid in experiments can lead to incorrect data interpretation and safety hazards.
Worked Examples
-
Naming a Binary Acid
- Formula: HBr
- Step 1: Identify the non-metal (bromine)
- Step 2: Apply the naming pattern: hydro- + bromine + -ic acid
- Answer: hydrobromic acid
-
Naming an Oxyacid
- Formula:
- Step 1: Identify the polyatomic ion ( is nitrate)
- Step 2: Convert -ate to -ic: nitric acid
- Answer: nitric acid
-
Naming a Base
- Formula:
- Step 1: Identify the metal (iron) and its oxidation state (3+)
- Step 2: Combine with hydroxide
- Answer: iron(III) hydroxide
Example Exercises with Solutions
-
MnO₂
- Solution: Manganese dioxide
-
Cu₂O
- Solution: Copper(I) oxide
-
Zn(OH)₂
- Solution: Zinc hydroxide
-
HI
- Solution: Hydroiodic acid
-
H₂CO₃
- Solution: Carbonic acid
Understanding proper naming conventions in chemistry reduces errors and enhances scientific communication, providing a solid foundation for further studies and practical applications.
500K+ Students Use These Powerful Tools to Master Naming Inorganic Compounds For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
111 flashcards
Flashcards on Naming Inorganic Compounds
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards8 quizzes
Quizzes on Naming Inorganic Compounds
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes63 questions
Exam questions on Naming Inorganic Compounds
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Naming Inorganic Compounds
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Naming Inorganic Compounds
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Naming Inorganic Compounds you should explore
Discover More Revision Notes Related to Naming Inorganic Compounds to Deepen Your Understanding and Improve Your Mastery
Load more notes