Photo AI
Last Updated Sep 24, 2025
Buffers in Chemistry Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Buffers in Chemistry quickly and effectively.
247+ students studying
Buffers in Chemistry
Buffers play a critical role in both biological and industrial systems by maintaining pH levels and preventing abrupt changes. An understanding of buffers is essential for various applications.
Buffer Fundamentals
-
Buffer Solution: Buffers are solutions that resist changes in pH when small amounts of acids or bases are introduced. They act as stabilisers within systems.
-
Importance:
- Biological Applications: Buffers help maintain enzyme activity, which is crucial for homeostasis. For example, blood buffer systems maintain a pH of approximately 7.4.
- Industrial Applications: In processes like food preservation and pharmaceuticals, buffers ensure both safety and efficiency.
Buffer Solution: Buffers resist pH changes when acids or bases are added.
Differentiation Between Acidic and Basic Buffers
-
Acidic Buffers:
- Composition: Comprised of a weak acid and its conjugate base.
- Applications: Utilised in food preservation and pharmaceuticals.
-
Basic Buffers:
- Composition: Consist of a weak base and its conjugate acid.
- Applications: Commonly found in cleaning solutions.
Common Misconceptions: Not all acidic/basic mixtures serve as effective buffers. The correct combinations are crucial.
Illustration of Buffer Action with Equations
- General Role: Buffers mitigate the impact of added H⁺ or OH⁻ ions.
- Reaction with added H⁺:
- Equation:
- Explanation: Acetate ions convert to acetic acid, minimising pH changes.
- Reaction with added OH⁻:
- Equation:
- Explanation: Acetic acid reacts to form water, stabilising the pH.
- Reaction with added H⁺:
Buffers diminish pH fluctuations; they do not maintain a constant pH.
Diagram Demonstration
-
Diagram shows the pH range where buffers are most effective:
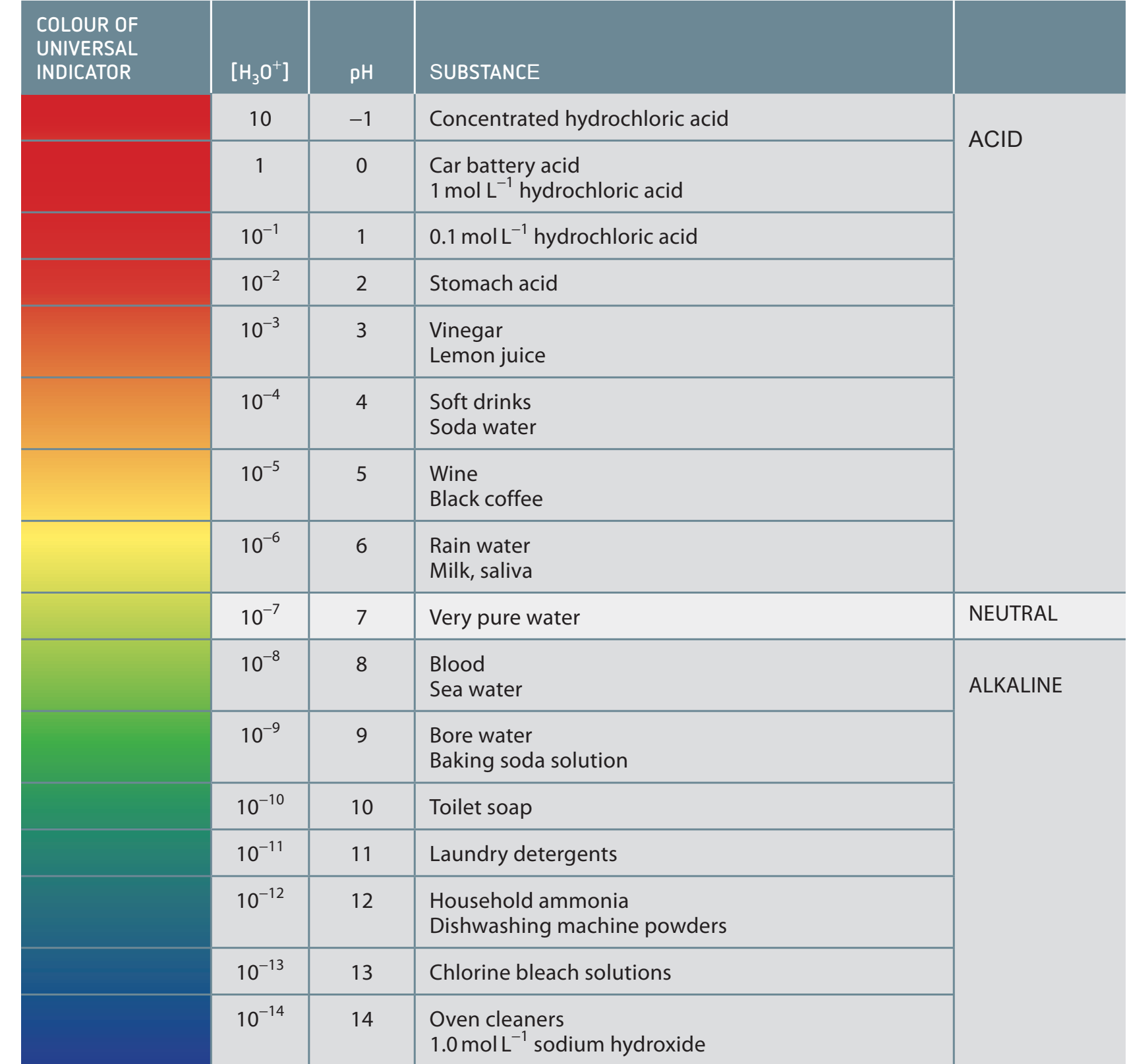
Buffer effectiveness depends on the ratio of acid to base, affecting pH stability.
Henderson-Hasselbalch Equation
Equation Overview:
-
A fundamental formula for calculating buffer pH:
-
Derivation: Derivation includes acid dissociation and the equilibrium constant to relate pH.
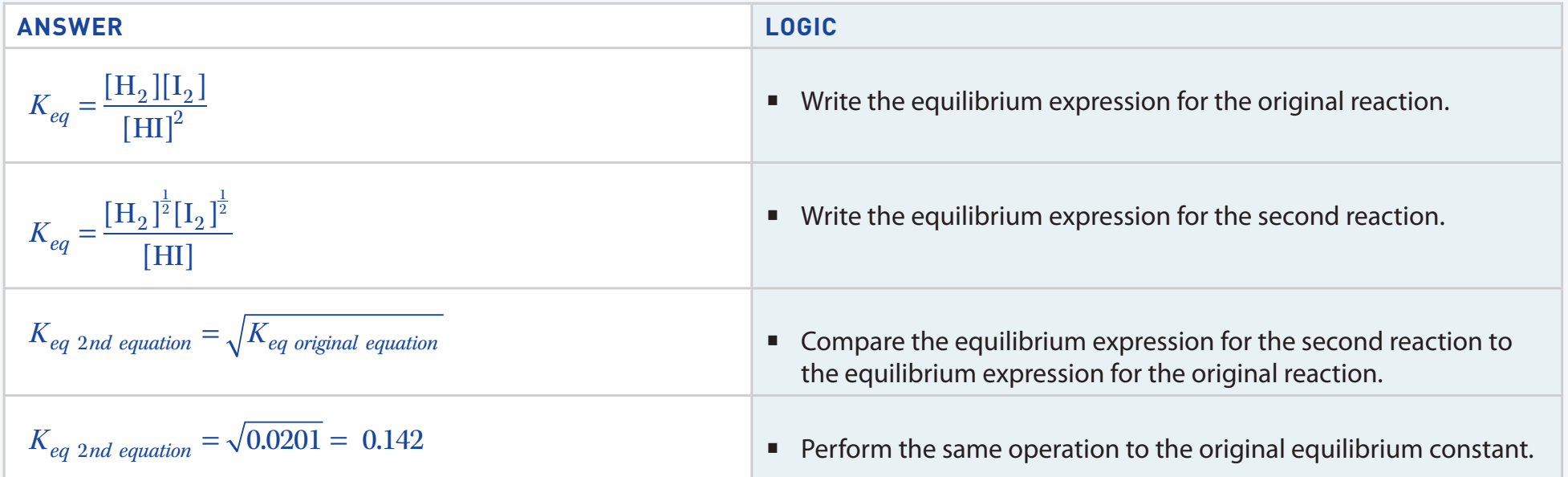
Worked Examples
Acetic Acid/Sodium Acetate Buffer Calculation:
- Problem: Calculate the pH of a buffer containing 0.1 M acetic acid () and 0.1 M sodium acetate () where of acetic acid is 4.76.
- Solution:
- Using the Henderson-Hasselbalch equation:
- Therefore,
Ammonia/Ammonium Chloride Buffer:
- Problem: Calculate the pH of a buffer containing 0.2 M ammonia () and 0.2 M ammonium chloride () where of ammonia is 4.75.
- Solution:
- First, calculate pOH:
- Then calculate pH:
Practical Experiment Guide
- Goal: Evaluate the efficiency and determine the limits of a buffer.
- Procedure:
- Prepare the buffer for testing.
- Calibrate the pH meter.
- Measure the initial pH.
- Incrementally add an acid or base and record the pH changes.
Ensure all safety equipment is worn when handling acids and bases.
Importance of Buffers in Natural Systems
Biological Systems
- Blood pH Regulation: Essential for preventing conditions like acidosis and alkalosis.
Bicarbonate Buffer System: Regulates blood pH by neutralising excess acids or bases.
Environmental Systems
-
Ocean Buffer Capacity: Maintains the pH balance, which is critical for marine ecosystems.

-
Impact of CO₂ Rise: Causes ocean acidification, affecting ecosystems like the Great Barrier Reef.
Adaptation Strategies and Future Research
- Biological Adaptations: Some coral species and plants adapt through genetic changes.
Research investigates how organisms adapt to buffer pH changes.
500K+ Students Use These Powerful Tools to Master Buffers in Chemistry For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
176 flashcards
Flashcards on Buffers in Chemistry
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards20 quizzes
Quizzes on Buffers in Chemistry
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes45 questions
Exam questions on Buffers in Chemistry
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Buffers in Chemistry
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Buffers in Chemistry
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Buffers in Chemistry you should explore
Discover More Revision Notes Related to Buffers in Chemistry to Deepen Your Understanding and Improve Your Mastery
