Photo AI
Last Updated Sep 27, 2025
Properties of Metallic Substances Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Properties of Metallic Substances quickly and effectively.
344+ students studying
1.4.9 Properties of Metallic Substances
Properties of Metals Explained by Metallic Bonding
High melting and boiling points
The strong electrostatic attraction between the ions and delocalised electrons means that a lot of energy is required to break the metallic bonds, giving metals high melting and boiling points.
Electrical conductivity
Metals are good conductors of electricity because the delocalised electrons are free to move and carry an electric charge throughout the structure.
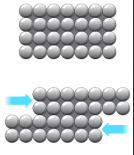
Malleability and ductility
The layers of ions in a metallic lattice can slide over each other without breaking the metallic bond. This makes metals malleable (can be hammered into shape) and ductile (can be drawn into wires).
Summary
Metallic bonding is characterised by the attraction between a lattice of positive ions and a sea of delocalised electrons. This bonding explains key properties of metals, such as high melting points, electrical conductivity, and malleability. The strength of metallic bonding is influenced by the charge and size of the metal ions.
500K+ Students Use These Powerful Tools to Master Properties of Metallic Substances For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
90 flashcards
Flashcards on Properties of Metallic Substances
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards9 quizzes
Quizzes on Properties of Metallic Substances
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Properties of Metallic Substances
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Properties of Metallic Substances
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Properties of Metallic Substances
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Properties of Metallic Substances you should explore
Discover More Revision Notes Related to Properties of Metallic Substances to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Types of Bonding & Properties
Changes in State: Energy Changes
490+ studying
187KViews