Photo AI
Last Updated Sep 27, 2025
Trends of Period 3 Elements: Atomic Radius Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Trends of Period 3 Elements: Atomic Radius quickly and effectively.
468+ students studying
2.1.2 Trends of Period 3 Elements: Atomic Radius
Explanation of Atomic Radius Trend
The atomic radius is the distance from the nucleus of an atom to the outermost electron shell. Across Period 3 of the Periodic Table (from sodium, , to argon, ), the atomic radius decreases steadily.
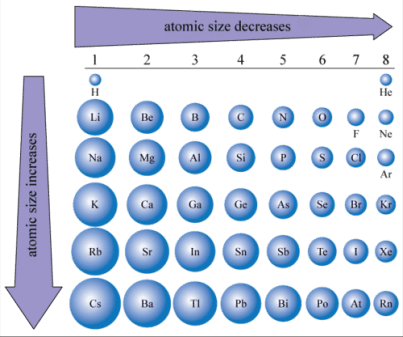
Reasons for the Trend
Increase in Nuclear Charge
As you move across the period from left to right, the number of protons in the nucleus increases, leading to a higher nuclear charge. This greater positive charge in the nucleus exerts a stronger electrostatic attraction on the electrons, pulling them closer to the nucleus.
The Same Number of Electron Shells
Despite the increase in nuclear charge, the electrons across Period 3 elements are all added to the same energy level (third shell). There is no increase in shielding because there are no additional electron shells between the nucleus and the outermost electrons, so the stronger nuclear charge pulls the electrons closer to the nucleus.
Greater Attraction to Nucleus
As a result of the increasing nuclear charge and consistent shielding, the outer electrons are pulled closer to the nucleus, reducing the atomic radius.
Summary
- Across Period 3, from to , the atomic radius decreases due to the increasing nuclear charge and the same number of electron shells.
500K+ Students Use These Powerful Tools to Master Trends of Period 3 Elements: Atomic Radius For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
40 flashcards
Flashcards on Trends of Period 3 Elements: Atomic Radius
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards4 quizzes
Quizzes on Trends of Period 3 Elements: Atomic Radius
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Trends of Period 3 Elements: Atomic Radius
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Trends of Period 3 Elements: Atomic Radius
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Trends of Period 3 Elements: Atomic Radius
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Trends of Period 3 Elements: Atomic Radius you should explore
Discover More Revision Notes Related to Trends of Period 3 Elements: Atomic Radius to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Periodicity
Trends of Period 3 Elements: First Ionisation Energy
270+ studying
184KViews