Photo AI
Last Updated Sep 27, 2025
Modification of Alkanes by Cracking Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Modification of Alkanes by Cracking quickly and effectively.
234+ students studying
3.2.2 Modification of Alkanes by Cracking
What is Cracking?
Cracking is a process used to break the carbon-carbon () bonds in long-chain alkanes, converting them into shorter-chain alkanes and alkenes. This process is crucial because there is a higher demand for shorter hydrocarbons, which are more useful in industries such as fuel production and plastics.
Types of Cracking
There are two main types of cracking processes: thermal cracking and catalytic cracking.
Thermal Cracking
- Conditions: High pressure and very high temperature (around 700-1200 K).
- Products: A high percentage of alkenes (e.g., ethene, which is used to produce polymers).
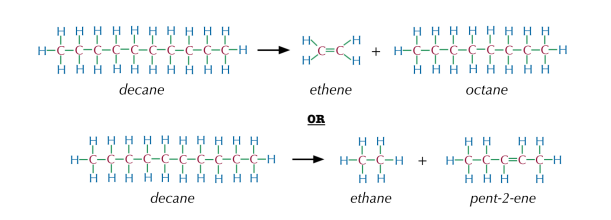
- Mechanism: Involves breaking the bonds in a random manner, resulting in a variety of smaller hydrocarbons. Example:
The cracking of decane () might produce ethene () and octane ():
Catalytic Cracking
- Conditions: Lower pressure, high temperature (around 720 K), and the presence of a zeolite catalyst.
- Products: Motor fuels (e.g., gasoline) and aromatic hydrocarbons.
- Mechanism: The catalyst speeds up the breaking of bonds, focusing on producing branched alkanes and aromatic compounds, which are important in motor fuel production.
2. Economic Reasons for Cracking
Cracking is economically important because it helps meet the demand for more useful hydrocarbons. Here's why cracking is essential:
High Demand for Short-Chain Hydrocarbons
- Short-chain alkanes are better fuels because they are more volatile and burn more easily. For example, propane and butane are widely used in heating and cooking.
- Short-chain alkenes are more reactive than alkanes and are used as feedstock for producing polymers like plastics.
Imbalance in Supply
- Fractional distillation of crude oil naturally produces a surplus of long-chain hydrocarbons and fewer short-chain hydrocarbons than are needed. Long-chain hydrocarbons are less useful and harder to ignite, making them less desirable for fuel. By converting the excess of long-chain hydrocarbons into more useful short-chain alkanes and alkenes, cracking maximises the utility of crude oil and aligns supply with industrial demand.
Summary
Cracking is a vital process in the petroleum industry, ensuring that the right balance of hydrocarbon products is available for various uses, from fuels to the production of plastics.
500K+ Students Use These Powerful Tools to Master Modification of Alkanes by Cracking For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
40 flashcards
Flashcards on Modification of Alkanes by Cracking
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards4 quizzes
Quizzes on Modification of Alkanes by Cracking
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Modification of Alkanes by Cracking
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Modification of Alkanes by Cracking
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Modification of Alkanes by Cracking
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Modification of Alkanes by Cracking you should explore
Discover More Revision Notes Related to Modification of Alkanes by Cracking to Deepen Your Understanding and Improve Your Mastery
