Photo AI
Last Updated Sep 27, 2025
Alcohol Production Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Alcohol Production quickly and effectively.
491+ students studying
3.5.2 Alcohol Production
This note covers the production of ethanol, a crucial alcohol in chemistry and industry, by two primary methods: fermentation and hydration of alkenes. Additionally, the note explores the use of ethanol as a biofuel and its environmental implications.
Introduction to Ethanol Production
Ethanol is an important industrial chemical used as a solvent, in pharmaceuticals, and as a biofuel. There are two main ways to produce ethanol on an industrial scale:
- Fermentation of glucose: A renewable method using biological processes.
- Hydration of ethene: A faster, continuous process using petrochemical resources.
Method 1: Fermentation of Glucose
Process Overview
Fermentation is a biological process in which glucose is converted to ethanol and carbon dioxide. This occurs through anaerobic respiration by yeast.
Chemical Equation:
Key Characteristics of the Process:
- Type: Batch process (done in separate, limited quantities).
- Renewable: Utilises crops like sugar cane, maize, and sugar beet.
Conditions for Fermentation
- Temperature: 35°C – A compromise temperature that ensures a reasonable reaction rate without denaturing yeast enzymes.
- Pressure: 1 atm – Normal atmospheric pressure is sufficient.
- Catalyst: Enzymes present in yeast.
- Other Requirements: Anaerobic conditions to avoid the oxidation of ethanol to ethanoic acid.
Advantages and Disadvantages
- Advantages:
- Renewable raw materials.
- Low energy requirements due to moderate temperature.
- Disadvantages:
- Slow reaction rate compared to chemical processes.
- High labour costs, although equipment costs are relatively low.
- Impure product requiring further purification by fractional distillation.
- Moderate yield due to enzyme inhibition at high ethanol concentrations.
Fractional Distillation for Purification
Since the ethanol from fermentation contains water, it needs purification:
- Process: Fractional distillation exploits the different boiling points of ethanol (78°C) and water (100°C).
- Outcome: Pure ethanol is collected after vaporising first during distillation.
Method 2: Hydration of Ethene
Process Overview
Hydration of ethene is a chemical process in which water is added to ethene, producing ethanol. This method is faster and more efficient compared to fermentation.
Chemical Equation:
Mechanism for the Hydration of Ethene
Hydration of ethene involves an electrophilic addition mechanism:
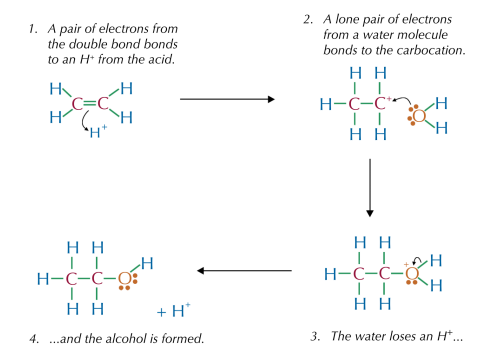
- The double bond in ethene acts as a nucleophile, attacking a proton () from the acid catalyst, forming a carbocation.
- Water () then attacks the carbocation, leading to the formation of a protonated alcohol.
- The catalyst () is regenerated as a hydrogen ion is released, producing ethanol.
Key Characteristics of the Process:
- Type: Continuous process (steady and efficient production).
- Non-Renewable: Uses ethene derived from crude oil.
Conditions for Hydration
- Temperature: 300°C – High temperature to ensure a fast reaction.
- Pressure: 60 atm – High pressure to increase the reaction rate.
- Catalyst: Concentrated phosphoric acid () or sulfuric acid ().
Advantages and Disadvantages
- Advantages:
- Continuous and efficient, with low labour costs.
- High purity of ethanol without the need for distillation.
- Fast reaction rate, leading to nearly 100% yield.
- Disadvantages:
- High energy requirements due to elevated temperature and pressure.
- Relies on finite petrochemical resources.
Ethanol as a Biofuel
Definition of a Biofuel
A biofuel is a fuel derived from living organisms or recently living biomass, such as plants. Ethanol from fermentation is considered a biofuel because it comes from renewable crops.
Carbon Neutrality Debate
Ethanol is often labelled "carbon-neutral" because the carbon dioxide released during its combustion is theoretically balanced by the absorbed by crops during photosynthesis.
Combustion of Ethanol Equation:
Arguments For and Against Carbon Neutrality
- For: Plants used for ethanol absorb from the atmosphere, offsetting emissions when ethanol burns.
- Against: Additional emissions arise from farming, transportation, and ethanol processing, challenging the carbon-neutral claim.
Environmental and Ethical Considerations of Biofuel Use
Benefits of Biofuels
- Sustainability: Reduces reliance on fossil fuels if grown sustainably.
- Lower Carbon Footprint: Potentially reduces greenhouse gas emissions compared to traditional fuels.
Challenges of Biofuels
- Food vs Fuel Dilemma: Using food crops for biofuel can reduce food availability, leading to higher food prices.
- Deforestation and Land Use: Large-scale cultivation of biofuel crops can lead to deforestation, loss of biodiversity, and soil degradation.
- Water Consumption: Crop irrigation requires significant water, which may strain local resources, especially in areas prone to drought.
By understanding both fermentation and hydration processes, you can assess the best method for ethanol production based on efficiency, cost, and environmental impact. This awareness is crucial in the context of sustainable energy and industrial chemistry.
500K+ Students Use These Powerful Tools to Master Alcohol Production For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
40 flashcards
Flashcards on Alcohol Production
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards4 quizzes
Quizzes on Alcohol Production
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Alcohol Production
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Alcohol Production
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Alcohol Production
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Alcohol Production you should explore
Discover More Revision Notes Related to Alcohol Production to Deepen Your Understanding and Improve Your Mastery
