Photo AI
Last Updated Sep 27, 2025
pH Curves Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand pH Curves quickly and effectively.
311+ students studying
5.6.4 pH Curves
Types of pH Curves
There are four primary types of pH curves, each shaped by the combination of acids and bases used. Recognizing these curves is essential for interpreting titration results accurately.
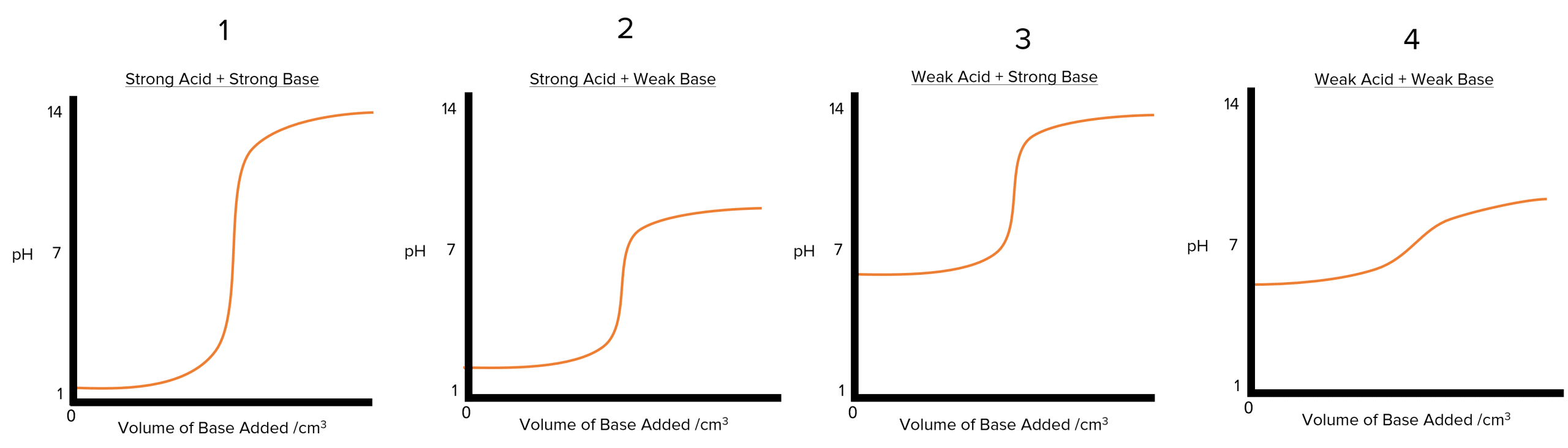
- Strong Acid with Strong Base: The pH starts low (acidic) and increases sharply near the equivalence point, finishing in a basic range.
- Strong Acid with Weak Base: The curve starts low, with a moderate pH increase and a smaller, gradual shift around the equivalence point.
- Weak Acid with Strong Base: The pH starts higher (weakly acidic) and rises gradually, with a less steep change at the equivalence point.
- Weak Acid with Weak Base: The pH change around the equivalence point is very gradual, and the solution does not reach strong acidic or basic levels.
Determining Acid or Base Added
- If the pH at the start is below 7, a base is being added to an acid.
- If the pH at the start is above 7, an acid is being added to a base.
Equivalence Point and Indicator Selection
The equivalence point depends on the acid and base used:
- Strong Acid–Strong Base: Equivalence point near pH 7, suitable for most indicators.
- Weak Acid–Strong Base: Equivalence point above pH 7; phenolphthalein is preferred.
- Strong Acid–Weak Base: Equivalence point below pH 7; methyl orange is suitable. Understanding curves and choosing the correct indicator ensures accurate endpoint detection.
Plotting pH Curves in Experiments
You can plot pH curves to visualize how pH changes during a titration.
To do this:
- Use a pH meter to record the initial pH of the solution.
- Add the titrant in small increments, recording the pH after each addition.
- Plot the pH against the volume added to reveal the titration curve and identify the equivalence point.
500K+ Students Use These Powerful Tools to Master pH Curves For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
50 flashcards
Flashcards on pH Curves
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards5 quizzes
Quizzes on pH Curves
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on pH Curves
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on pH Curves
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on pH Curves
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to pH Curves you should explore
Discover More Revision Notes Related to pH Curves to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Further Acids & Bases Calculations (A Level only)
Acid Dissociation Constant
258+ studying
192KViews96%
114 rated
Further Acids & Bases Calculations (A Level only)
Titrations
357+ studying
191KViews