Photo AI
Last Updated Sep 27, 2025
Shapes of Complex Ions Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Shapes of Complex Ions quickly and effectively.
401+ students studying
6.2.3 Shapes of Complex Ions
Shapes of Complex Ions
Transition metal ions form complex ions with characteristic shapes based on the size and type of ligands bonded to the metal. These shapes influence the properties, stability, and stereochemistry of the complex ions.
Octahedral Complexes
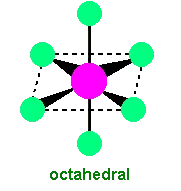
Octahedral complexes have six ligands symmetrically arranged around the central metal ion.
Typical Ligands:
Small ligands like water () and ammonia () often form octahedral complexes with transition metals.
Isomerism in Octahedral Complexes:
Cis–Trans Isomerism:
-
Occurs when two identical ligands are adjacent (cis) or opposite (trans) to each other.
-
This is a special form of E–Z isomerism. Optical Isomerism:
-
When three bidentate ligands form a chiral complex that cannot be superimposed on its mirror image, creating optical isomers.
Example Complex:
Example: shows cis-trans isomerism with and ligands.
Tetrahedral Complexes
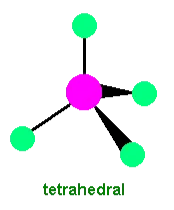
Tetrahedral complexes form when four ligands surround a central metal ion in a tetrahedral shape.
Typical Ligands:
Larger ligands, such as chloride ions (), commonly lead to tetrahedral coordination due to steric effects.
Isomerism in Tetrahedral Complexes:
Tetrahedral complexes do not exhibit cis–trans or optical isomerism due to their symmetrical structure.
Example Complex:
Example: , a copper(II) complex with chloride ligands, typically adopts a tetrahedral shape.
Square Planar Complexes

Square planar complexes have four ligands arranged in a square around the central metal ion, commonly seen in complexes of metals like platinum and palladium.
Isomerism in Square Planar Complexes:
Cis–Trans Isomerism:
- Just like octahedral complexes, square planar complexes with two pairs of identical ligands exhibit cis–trans isomerism.
Example of Cis Isomer: Cisplatin, , a square planar complex with platinum, is used in cancer treatment.
Only the cis isomer is effective as a drug because of its ability to interact with DNA.
Linear Complexes
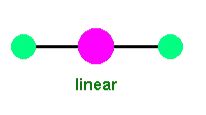
Linear complexes form when two ligands are bonded to a central metal ion, resulting in a straight-line arrangement.
Example Complex:
Example: The silver complex , found in Tollens' reagent, is linear.
Tollens' reagent is used to test for aldehydes, which reduce to metallic silver.
Stereoisomerism in Complex Ions
Stereoisomerism arises when complexes have the same formula but different spatial arrangements.
There are two main types of stereoisomerism:
- Cis–Trans Isomerism: Present in octahedral and square planar complexes where identical ligands can either be adjacent (cis) or opposite (trans).
- Optical Isomerism: Seen in chiral octahedral complexes with bidentate ligands. Optical isomers are non-superimposable mirror images, similar to left and right hands, and rotate plane-polarized light in opposite directions.
Summary of Shapes and Isomerism
Shapes of Complex Ions
| Shape | Common Ligands | Example Complex | Isomerism |
|---|---|---|---|
| Octahedral | Cis–Trans, Optical | ||
| Tetrahedral | None | ||
| Square Planar | (cisplatin) | Cis–Trans | |
| Linear | None |
500K+ Students Use These Powerful Tools to Master Shapes of Complex Ions For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
70 flashcards
Flashcards on Shapes of Complex Ions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards7 quizzes
Quizzes on Shapes of Complex Ions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Shapes of Complex Ions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Shapes of Complex Ions
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Shapes of Complex Ions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Shapes of Complex Ions you should explore
Discover More Revision Notes Related to Shapes of Complex Ions to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Transition Metals (A Level only)
General Properties of Transition Metals
271+ studying
199KViews96%
114 rated
Transition Metals (A Level only)
Formation of Coloured Ions
286+ studying
197KViews96%
114 rated
Transition Metals (A Level only)
Variable Oxidation States
444+ studying
182KViews