Photo AI
Last Updated Sep 27, 2025
Racemic Mixtures Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Racemic Mixtures quickly and effectively.
494+ students studying
7.1.3 Racemic Mixtures
A common exam question involves racemic mixtures, so make sure to revise them!
These occur when a molecule contains a chiral centre, yet the mixture is optically inactive, which may seem contradictory to what we've just discussed about chirality and optical activity! A racemic mixture contains equal amounts of both enantiomers (50:50 mixture)
Since a racemic mixture contains equal amounts of both enantiomers, their optical activities cancel each other out. For instance, one enantiomer may rotate plane-polarised light by +10 degrees, while the other enantiomer rotates it by -10 degrees. The net result is 0 degrees, making it appear as if the substance is optically inactive or lacks a chiral centre.
You may be wondering why this happens, and it's a common exam question. The explanation lies in the way these molecules are formed.
Racemic mixtures are typically produced through:
- Nucleophilic addition to ketones or aldehydes
- Reactions involving a carbocation intermediate
Below is an example of nucleophilic attack on a carbonyl group, a reaction that often results in the formation of a racemic mixture.
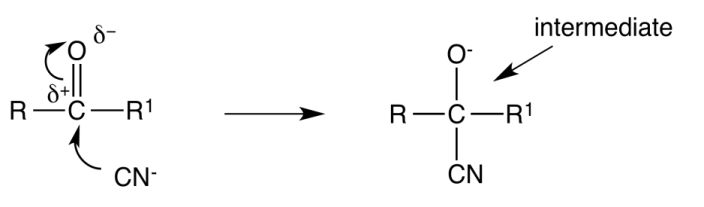
The crucial concept to understand is that the carbonyl group is planar (flat). Because of this, the nucleophile can attack the carbonyl carbon from either the top or the bottom of the plane. This leads to the production of two enantiomers.
The only way to distinguish between optical isomers is through their optical activity. However, a racemic mixture contains a 50:50 ratio of both enantiomers, meaning their opposite optical effects cancel each other out, making the mixture optically inactive.
Since the nucleophile has an equal probability of attacking from above or below the plane, a 50:50 mix of enantiomers is formed in the reaction, resulting in the racemic mixture.
Racemates in Pharmaceuticals
In drug manufacture, it is sometimes difficult or expensive to produce a single enantiomer. As a result, racemic mixtures may be used. However, if only one enantiomer is effective, the racemate might be optically inactive and may reduce drug efficacy or cause unwanted side effects.
500K+ Students Use These Powerful Tools to Master Racemic Mixtures For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
30 flashcards
Flashcards on Racemic Mixtures
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards3 quizzes
Quizzes on Racemic Mixtures
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Racemic Mixtures
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Racemic Mixtures
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Racemic Mixtures
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Racemic Mixtures you should explore
Discover More Revision Notes Related to Racemic Mixtures to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Optical Isomerism (A-level only)
Identifying Chiral Centres
325+ studying
192KViews