Photo AI
Last Updated Sep 27, 2025
Thin Layer Chromatography Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Thin Layer Chromatography quickly and effectively.
479+ students studying
7.11.2 Thin Layer Chromatography
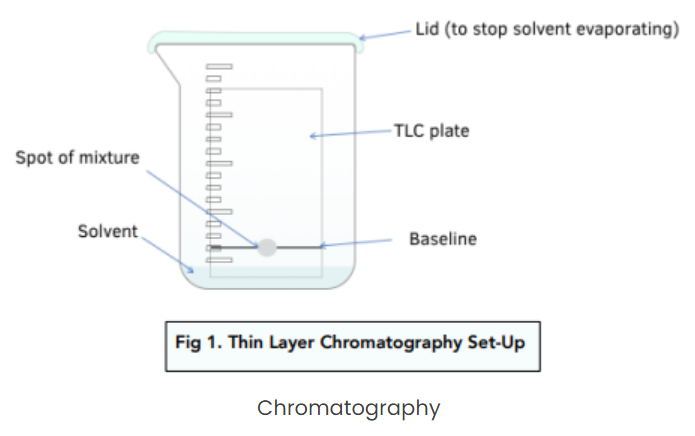
The components of a thin layer chromatography (TLC) setup include:
- Stationary Phase: A layer of alumina (aluminium oxide) or silica (silicon oxide) gel, spread across a plate made of metal, glass, or plastic.
- Mobile Phase: An organic solvent in which the plate is placed for the chromatography process. To perform TLC, a small spot of the sample mixture is applied near the bottom of the plate, which is then placed in a sealed container containing the solvent.
The solvent (mobile phase) moves up the plate by capillary action, passing through the stationary phase.
As this happens, the molecules in the mixture interact differently with the mobile and stationary phases based on their relative affinities or attractions for each phase:
- Molecules with a stronger attraction to the mobile phase travel more quickly up the plate.
- Molecules with a stronger attraction to the stationary phase move more slowly up the plate. After the solvent has moved up the plate, the different substances can be identified by examining the positions of the spots and calculating their Rf values (retention factors).
Step-by-step
- On the TLC plate, mark the origin and put a cross at the centre of the line using a pencil.
- Using a capillary tube, lift some of the sample dissolved in the appropriate solvent.
- Dap onto the cross you made.
- Place the thin-layer chromatogram in the same solvent (with the origin above the solvent's surface).
- Allow chromatogram to run - check it every few mins to ensure that the solvent has not run off the top end of the chromatogram.
- As the solvent moves, the substances in the spot also move up and separate.
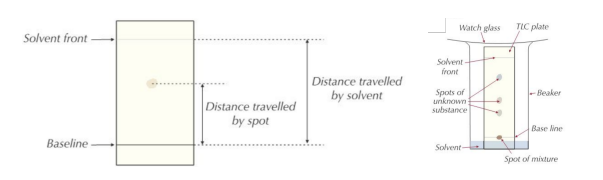
- Once finished, draw a pencil line to show where the solvent has reached on the chromatogram.
- This line (top point) is called the solvent front.
- Measure the distance moved by the spot and by the solvent.
- Calculate the Rf value. Rf = distance moved by spot / distance moved by solvent
Developing Chromatograms
- Most chromatograms are not visible unless they are developed.
- Some spots on chromatograms are only visible under UV light.
- These can be marked with a pencil for viewing in normal light.
- Others (mainly aromatic compounds) require a solution of iodine to mark.
500K+ Students Use These Powerful Tools to Master Thin Layer Chromatography For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
50 flashcards
Flashcards on Thin Layer Chromatography
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards5 quizzes
Quizzes on Thin Layer Chromatography
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Thin Layer Chromatography
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Thin Layer Chromatography
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Thin Layer Chromatography
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Thin Layer Chromatography you should explore
Discover More Revision Notes Related to Thin Layer Chromatography to Deepen Your Understanding and Improve Your Mastery
