Photo AI
Last Updated Sep 27, 2025
Nucleophilic Addition Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Nucleophilic Addition quickly and effectively.
226+ students studying
7.2.3 Nucleophilic Addition
Aldehydes and Ketones partake in nucleophilic addition reactions, some examples of nucleophilic addition reactions include:
- The reduction of aldehydes to primary alcohols, and ketones to secondary alcohols, using the reducing agent in aqueous solution.
- The reaction of the ion with aldehydes and ketones to produce hydroxynitriles (, followed by dilute acid)
Nucleophilic Addition Reactions of Carbonyl Compounds
Nucleophilic addition is an important mechanism in organic chemistry where a nucleophile (electron-rich species) adds to a carbonyl compound, such as an aldehyde or ketone.
- When carbonyl compounds react with potassium cyanide () followed by dilute acid, hydroxynitriles are formed. This reaction is significant in synthesis as it extends the carbon chain and introduces functional groups that can undergo further reactions.
Here's a comprehensive breakdown of the nucleophilic addition of carbonyls with .
Nucleophilic Addition Mechanism
Carbonyl Group:
-
Carbonyl compounds (aldehydes and ketones) contain a polar bond.
-
The oxygen atom is more electronegative than carbon, creating a dipole with a partially positive carbon () and a partially negative oxygen (). Nucleophile:
-
In the presence of in aqueous solution, the cyanide ion () acts as a nucleophile, attracted to the carbon in the group.
Reaction Mechanism Steps
- Attack by Cyanide Ion ():
- The ion attacks the carbonyl carbon, opening up the double bond and forming a tetrahedral intermediate. This step creates a negatively charged oxygen.
- Protonation:
- The intermediate is protonated by ions from the dilute acid, forming a hydroxynitrile.
- Example of a product: If propanone () is the starting material, the product will be 2-hydroxy-2-methylpropanenitrile.
- Overall Reaction:
This reaction is particularly valuable in organic synthesis as it introduces both a hydroxyl () and a nitrile () group, enabling further functional group transformations.
Formation of Hydroxynitriles and Nomenclature
- The product of this reaction is known as a hydroxynitrile.
- Naming: The nitrile group takes priority, giving the suffix "-nitrile." The hydroxyl group is named with the prefix hydroxy-. For example:
- If the starting material is ethanal (), the product is 2-hydroxypropanenitrile.
Optical Isomerism and Formation of Enantiomers
- Chiral Centers: The addition of to a carbonyl group in an unsymmetrical carbonyl compound (e.g., an aldehyde or asymmetric ketone) forms a new chiral centre at the carbon where the is added.
- Enantiomers: Since the ion can attack the planar carbonyl group from either above or below, this leads to the formation of two possible products, known as enantiomers.
- Racemic Mixture:
- Both enantiomers are produced in equal amounts (50:50), resulting in a racemic mixture.
- Racemic mixtures have no net optical rotation since the two enantiomers rotate plane-polarized light in equal but opposite directions. This feature can be tested using polarimetry.
Hazards of Using Potassium Cyanide ()
Potassium cyanide is highly toxic and poses significant health risks. Here are the key points about handling :
- Toxicity: KCN is extremely poisonous; it inhibits cellular respiration by binding to iron in cytochrome enzymes.
- Handling Precautions:
- Always handle in a fume cupboard to avoid inhaling toxic vapors.
- Use protective gloves and eyewear.
- Dispose of any waste containing cyanide according to safety regulations.
- In Case of Exposure: Immediate first aid and emergency procedures are critical due to the high toxicity of cyanide compounds.
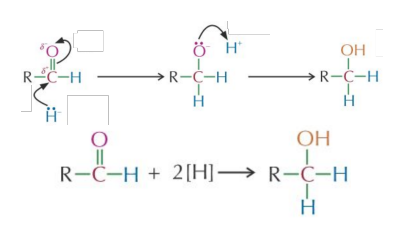
Reduction by Sodium Borohydride (NaBH4)
The is a complex anion but behaves like a hydride ion () So we draw it as a in the mechanism; that's the species acting as the nucleophile.
Reduction of aldehydes
Reagent: Aqueous followed by Conditions: Room temperature Mechanism: Nucleophilic = nucleophile Reaction type: Addition/Reduction Product: Primary alcohol
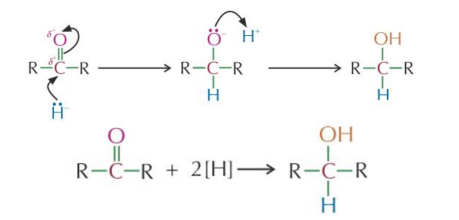
Reduction of ketones
Reagent: Aqueous followed by Conditions: Room temperature Mechanism: Nucleophilic = nucleophile Reaction type: Addition/Reduction Product: Secondary alcohol
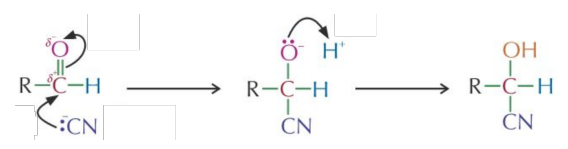
Reaction with Hydrogen Cyanide (HCN)
Reagent: - formed by reaction with followed by dilute
Conditions: Room temp.
Mechanism: Nucleophilic is the nucleophile
Reaction type: Addition
Product: Hydroxynitrile
Overall equations:
For aldehydes:
For ketones:
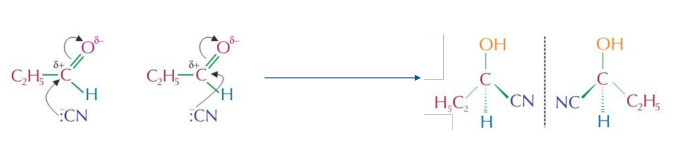
Formation of racemic mixtures of hydroxynitriles
- Aldehydes and unsymmetrical ketones form mixtures of enantiomers when they react with followed by dilute acid. • This is because the products of the reactions contain chiral carbon atoms.
- Nucleophilic addition reactions of , followed by dilute acid, can produce a racemic mixture of enantiomers which is optically inactive because:
- The C atom in the planar carbonyl group can be attacked by a nucleophile from above or below.
- There is an equal chance of attack from above or below. E.g. the products of the reaction propanal w/ potassium cyanide, under weakly acidic conditions are present as a racemic mixture.
500K+ Students Use These Powerful Tools to Master Nucleophilic Addition For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
30 flashcards
Flashcards on Nucleophilic Addition
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards3 quizzes
Quizzes on Nucleophilic Addition
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Nucleophilic Addition
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Nucleophilic Addition
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Nucleophilic Addition
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Nucleophilic Addition you should explore
Discover More Revision Notes Related to Nucleophilic Addition to Deepen Your Understanding and Improve Your Mastery
