Photo AI
Last Updated Sep 27, 2025
Acylation Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Acylation quickly and effectively.
455+ students studying
7.3.5 Acylation
Acylation is the process in which an acyl group () is introduced into a molecule. The acyl group consists of a carbonyl group () attached to an alkyl () or aryl () group. Acyl compounds like acyl chlorides, acid anhydrides, and amides play a crucial role in organic synthesis and reactivity due to their susceptibility to nucleophilic attack.
The structure of the acyl group is:
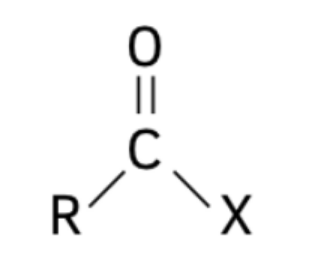
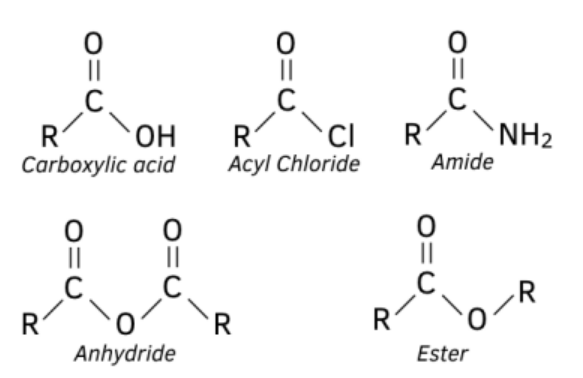
Structures of Acyl Compounds
- Acid Anhydrides:
- Structure: General formula is ''. Acid anhydrides consist of two acyl groups connected by an oxygen atom.
- Naming: Named by replacing "acid" in the carboxylic acid's name with "anhydride" (e.g., ethanoic anhydride).
- Symmetry: If both acyl groups are identical, the compound is symmetrical (e.g., ethanoic anhydride); if different, it is asymmetrical.
- Acyl Chlorides:
- Structure: General formula is . Acyl chlorides contain a carbonyl group attached to a chloride atom.
- Naming: Named by replacing "ic acid" in the carboxylic acid's name with "oyl chloride" (e.g., ethanoyl chloride).
- Reactivity: Highly reactive due to the electronegative chlorine, which makes the carbonyl carbon more susceptible to nucleophilic attack.
- Amides:
- Structure: General formula is , ', or , where the carbonyl group is attached to an amine group (.
- Naming: Named by replacing "ic acid" in the carboxylic acid's name with "amide" (e.g., ethanamide).
- Reactivity: Amides are generally less reactive towards nucleophiles than acyl chlorides and acid anhydrides because of resonance stabilization in the bond.
Polarization in Acyl Groups
In acyl groups, the carbonyl carbon () is polarized due to differences in electronegativity:
-
Electronegativity: Oxygen is more electronegative than carbon, creating a dipole in which oxygen is partially negative and carbon is partially positive
-
Nucleophilic Attack: The partial positive charge on the carbonyl carbon makes it susceptible to attack by nucleophiles (species that donate electron pairs). The degree of polarization varies depending on the substituent attached to the acyl group:
-
Acyl Chlorides: The highly electronegative chlorine atom withdraws electron density, increasing the partial positive charge on the carbonyl carbon, making acyl chlorides highly reactive.
-
Acid Anhydrides: The oxygen between the two acyl groups is less electronegative than chlorine, making acid anhydrides reactive but less so than acyl chlorides.
Nucleophilic Addition-Elimination Reactions of Acyl Chlorides and Acid Anhydrides
Acyl chlorides and acid anhydrides undergo nucleophilic addition-elimination reactions, where a nucleophile attacks the carbonyl carbon, resulting in the elimination of a leaving group.
- Reaction with Water (Hydrolysis): Acyl Chlorides:
Acyl chloride + water → carboxylic acid + hydrogen chloride Ethanoyl chloride with water:
- This is hydrolysis - acyl chlorides are susceptible to hydrolysis if any water is present.
- This means acid anhydrides are preferred for synthetic routes in industry. Mechanism:
- The oxygen atom in water acts as a nucleophile, attacking the carbonyl carbon of the acyl chloride.
- The addition of water to the carbonyl carbon leads to the formation of an unstable tetrahedral intermediate.
- The intermediate collapses, resulting in the elimination of hydrogen chloride () and formation of a carboxylic acid.
- Products: A carboxylic acid and hydrogen chloride ().
- Reaction Conditions: Very vigorous and often exothermic due to the high reactivity of acyl chlorides. Acid Anhydrides:
Mechanism:
- Water attacks one of the carbonyl carbons in the anhydride.
- The intermediate collapses, splitting the anhydride bond.
- The result is the formation of two molecules of carboxylic acid.
- Products: Two molecules of carboxylic acid.
- Reaction Conditions: Less vigorous than acyl chlorides, may require gentle heating.
- Reaction with Alcohols: Acyl chloride + alcohol → ester + hydrogen chloride Ethanoyl chloride with ethanol:
- This is a more effective method for the preparation of esters than esterification; acylation is complete whereas esterification is in a constant equilibrium. Acyl Chlorides:
Mechanism:
- Alcohol acts as the nucleophile, attacking the carbonyl carbon of the acyl chloride.
- A tetrahedral intermediate forms, which collapses to eliminate and produce an ester.
- Products: Ester and .
- Preference in Ester Formation: Acyl chlorides are often preferred for esterification with alcohols due to their reactivity. Using Acid Anhydrides
The acylation reactions of acid anhydrides are very similar to those of acyl chlorides, however:
- Acid anhydrides undergo slower and less vigorous reactions than acyl chlorides as they are less reactive.
- This is because the Cl- is a more powerful electron leaving group.
- Cl is more δ- (more electronegative) so it attracts the bonding pair of electrons towards itself, causing C to become more δ+
- This makes it more reactive
- Also, a carboxylic acid is formed as a by product, not HCl which is easier to deal with. Mechanism:
- Alcohol attacks the carbonyl carbon, leading to a breakdown of the anhydride.
- Produces an ester and a carboxylic acid. Products: Ester and carboxylic acid.
Reaction with Ammonia and Amines:
This reaction happens in 2 stages:
- Acyl chloride + ammonia → amide + hydrogen chloride Ethanoyl chloride with ammonia:
= nucleophile in 1st stage.
- Ammonia + hydrogen chloride → ammonium chloride i.e. NH3 + HCl → NH4+Cl-
NH3 = base in 2nd stage to neutralise the acidic by-product.
As a result 2 ammonia molecules react, SO this is the overall equation:
Acyl Chlorides:
With Ammonia:
acyl chloride + 2 ammonia → amide + ammonium chloride
-
Mechanism: Ammonia () attacks the carbonyl carbon, forming an intermediate that eliminates , resulting in an amide.
-
Products: Amide and ammonium chloride. With Primary Amines:
-
Mechanism: The primary amine attacks the acyl chloride, yielding a secondary amide and .
-
Products: Secondary amide and . This reaction happens in 2 stages:
- Acyl chloride + amine → N-substituted amide + HCL
- N-substituted amide: a compound where a hydrocarbon group has substituted one of the hydrogen atoms on the NH2 group.
- The 1st part of the name describes the hydrocarbon which has substituted the H atom.
Ethanoyl chloride with methylamine:
- Amine + hydrogen chloride → ammonium salt
Methylamine with hydrogen chloride:
As a result 2 amine molecules react - so the overall equation:
acyl chloride + 2 amine → n-substituted amide + ammonium salt
Acid Anhydrides:
- With Ammonia: Produces an amide and a carboxylic acid.
- With Amines: Forms an amide and a carboxylate salt.
Industrial Use: Making Aspirin
Acyl groups are frequently utilized in the synthesis of pharmaceuticals and other drugs because they can alter the properties of organic molecules, such as their solubility and stability. By introducing functional groups through acylation reactions, the effectiveness and bioavailability of drug molecules can be enhanced.
Aspirin, an ester, can be synthesized by reacting salicylic acid with either ethanoic anhydride or ethanoyl chloride:
Ethanoic Anhydride vs. Ethanoyl Chloride:
Advantages of Ethanoic Anhydride:
- It is cheaper and less corrosive.
- Produces ethanoic acid as a by-product, which is less hazardous than .
- Easier to control in reactions, as it is less reactive than ethanoyl chloride. The general structure of the different acyl groups you need to be familiar with.
500K+ Students Use These Powerful Tools to Master Acylation For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
60 flashcards
Flashcards on Acylation
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards6 quizzes
Quizzes on Acylation
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Acylation
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Acylation
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Acylation
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Acylation you should explore
Discover More Revision Notes Related to Acylation to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Carboxylic Acids & Derivatives (A-level only)
Carboxylic Acids
393+ studying
193KViews96%
114 rated
Carboxylic Acids & Derivatives (A-level only)
Ester Hydrolysis
246+ studying
182KViews