Photo AI
Last Updated Sep 27, 2025
Condensation Polymers Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Condensation Polymers quickly and effectively.
311+ students studying
7.6.1 Condensation Polymers
Polymer: a long chain of repeating units joined together. It is saturated. Monomer: unit which breaks its double bond to form a repeating unit; It is a small, unsaturated molecule. Condensation polymer: a polymer that is formed when monomers join together, eliminating a small molecule such as water (or ) in the process.
Condensation polymers are formed by reactions between:
- Dicarboxylic acids + diols, which forms a polyester. Dicarboxylic acids + diamines, which forms a polyamide (or polypeptide).
- The 'Di' prefix means "2 of"
- TWO groups on a Diol - TWO groups on a Dicarboxylic acid - TWO -groups on a Diamine Polyesters.
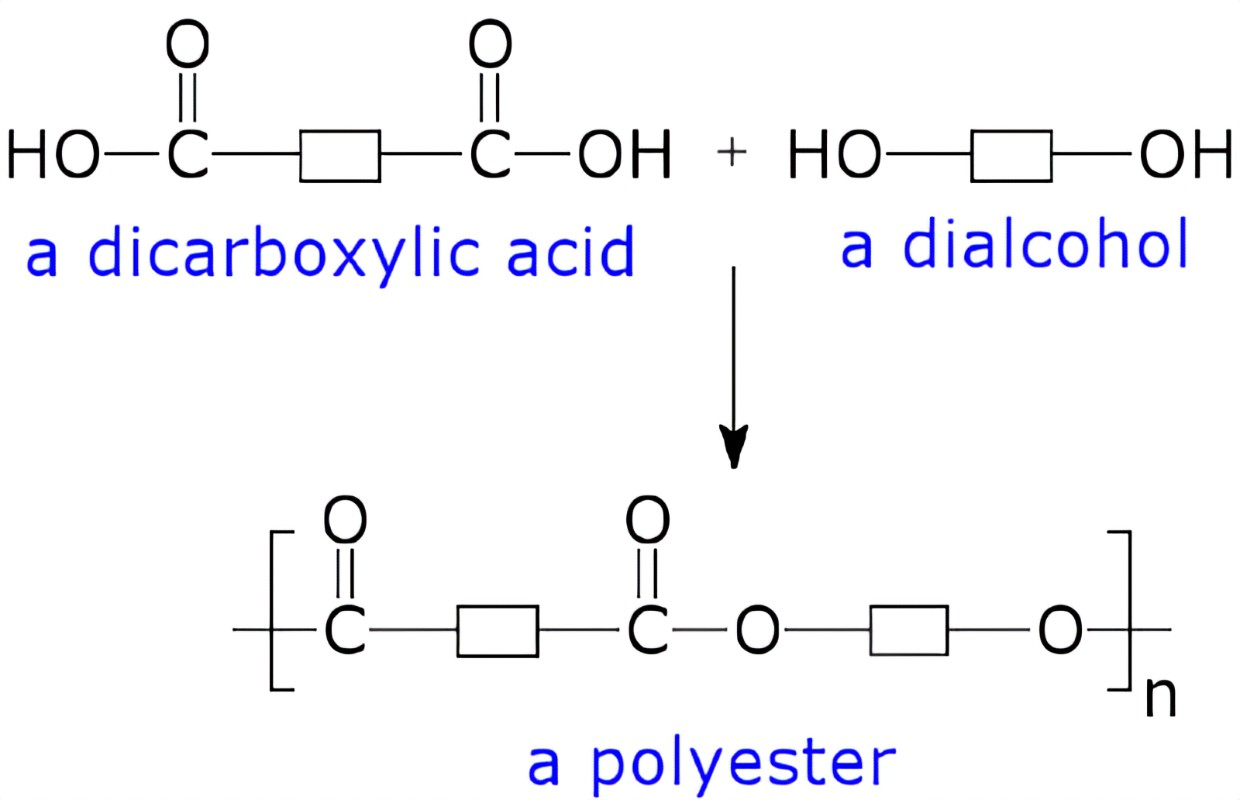
Reactions between dicarboxylic acids and diols make polyesters.
-
The carboxyl groups of dicarboxylic acid can react with the -OH groups of diols to form ester links.
-
A water molecule is lost each time an ester link is formed. Therefore, this is a condensation reaction.
-
There are permanent dipole-dipole attractions between the polymer chains in polyesters due to the the polar bonds in the ester linkage.
-
Therefore, there are greater forces of attraction between the polymer chains in polyesters compared to the weak van der Waals forces between addition polymer chains.
Terylene (a polyester)
- You need to know the makeup of 3 polymers
- One of these is a polyester and two are polyamides. This is the first one: Terylene.
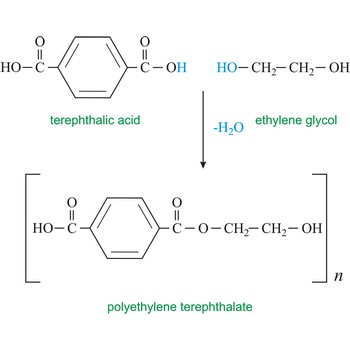
Terylene is used to make plastic bottles (and similar plastic products) and is recycable.
Polyamides
-
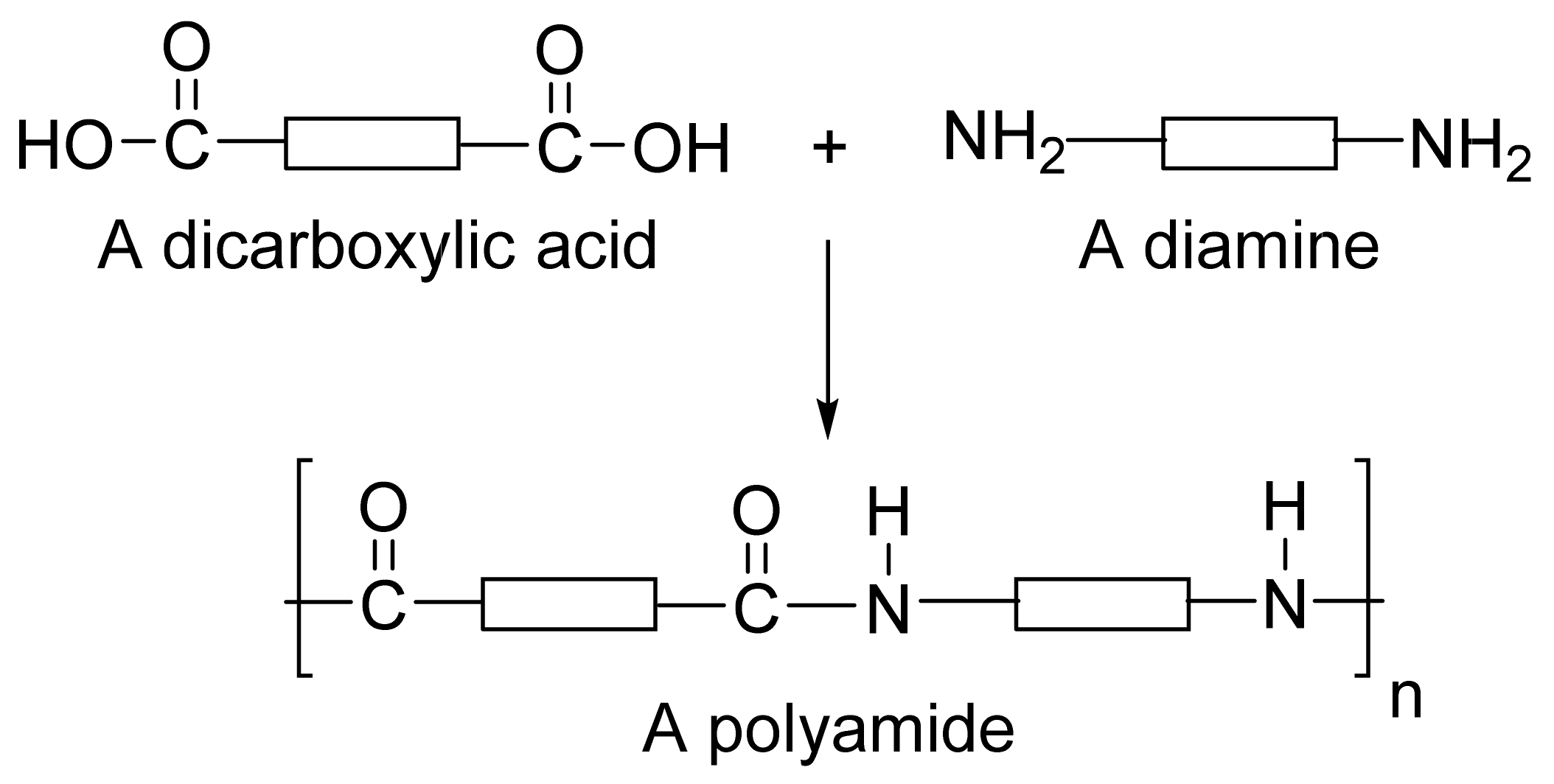
Reactions between dicarboxylic acids and diamines make polyamides.
-
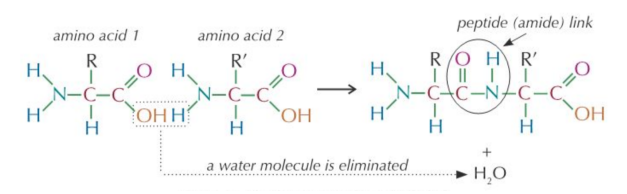
The carboxyl groups of dicarboxylic acid react with the diaminesamino groups to from amide links.
-
A water molecule is lost each time an amide link is formed, so this is a condensation reaction.
-
There are H-bonds between the polymer chains in polyamides due to the presence of bonds and bonds.
-
This means there are greater forces of attraction between the polymer chains in polyamides compared to the weak van der Waals forces between addition polymer chains.
Nylon 6,6 (a polyamide)
Monomer 1: a dicarboxylic acid, hexanedioic acid. Nonomer 2: a diamine, 1,6-diaminohexane.
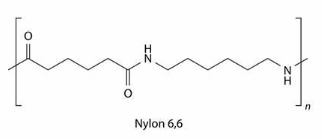
• Nylon is used to make fabrics, ropes, and medical prosthetics.
Kevlar (a polyamide)
Monomer 1: a dicarboxylic acid, benzene-1,4-dicarboxylic acid. Monomer 2: a diamine, 1,4-diaminobenzene.
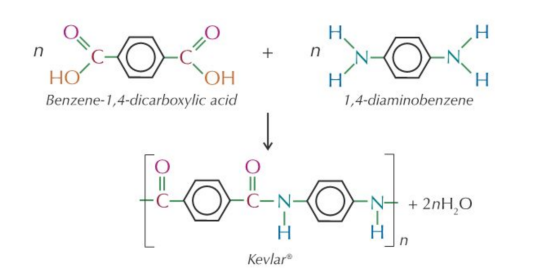
- Kevlar is used to make bullet-proof vests (because it's strong, stable and also fire resistant).
Polypeptides
- Amino acids (more on these in the amino acids topic) contain an aminefunctional group and a carboxylic acid functional group.
- These groups can react in condensation polymerisation to form polyamides
- They're more commonly called polypeptides when the monomers are amino acids rather than diamines and di-acids.
500K+ Students Use These Powerful Tools to Master Condensation Polymers For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
20 flashcards
Flashcards on Condensation Polymers
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards2 quizzes
Quizzes on Condensation Polymers
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Condensation Polymers
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Condensation Polymers
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Condensation Polymers
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Condensation Polymers you should explore
Discover More Revision Notes Related to Condensation Polymers to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Polymers (A Level only)
Biodegradability & Disposal of Polymers
215+ studying
180KViews