Photo AI
Last Updated Sep 27, 2025
Amino Acids Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Amino Acids quickly and effectively.
460+ students studying
7.7.1 Amino Acids
Structure and Chirality of Amino Acids
Amino acids are organic molecules that contain two essential functional groups: an amine group () and a carboxylic acid group (). Each amino acid follows a general structure:
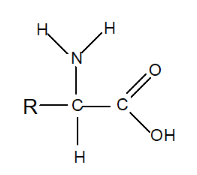
A central carbon atom (called the α-carbon) is bonded to four groups:
- A hydrogen atom ()
- A carboxylic acid group ()
- An amine group ()
- A variable R group, or side chain, that defines each unique amino acid. This structure makes most amino acids chiral, meaning they exist in two mirror-image forms (enantiomers) that are not superimposable. The exception is glycine, where the R group is simply a hydrogen atom, resulting in a non-chiral structure.
Amino acids are abundant in nature and serve numerous functions within the human body. Some straightforward examples include:
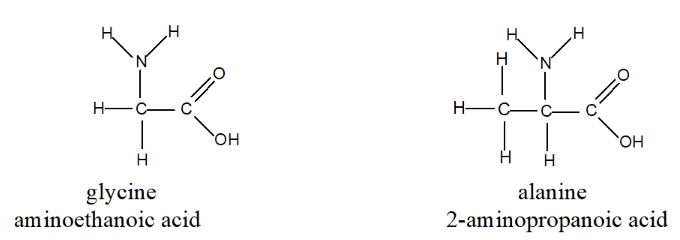
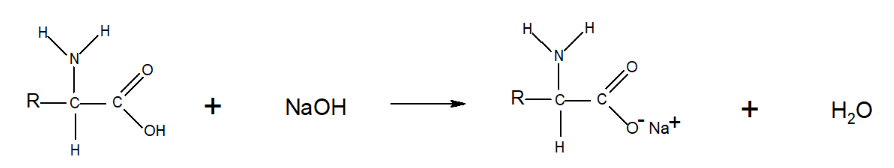
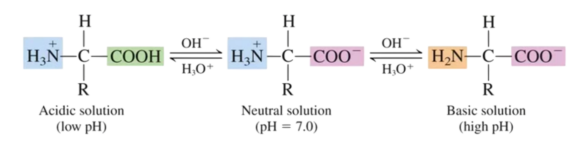
Amino acids react with strong bases such as sodium hydroxide:
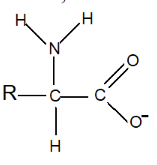
In high pH, therefore, amino acids exist in anionic form:
Reaction with acids:
Amino acids react with strong acids such as hydrochloric acid:
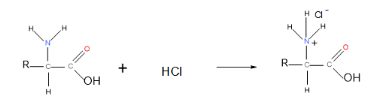
In low pH, therefore, amino acids exist in cationic form:
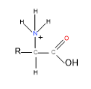
Reaction with itself
Since amino acids have a proton donating group and a proton accepting group on the same molecule, it follows that each molecule can undergo an acid-base reaction with itself:
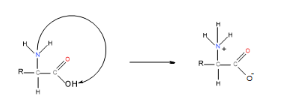
The ion formed from this reaction is known as a zwitterion, which occurs in the solid state.
Therefore, in the solid state, amino acids exist as ionic compounds, explaining their solid form and high melting points.
Acidic and Basic Properties of Amino Acids
The presence of both acidic (carboxylic acid group) and basic (amine group) functional groups means that amino acids can act as both acids and bases:
- The carboxylic acid group () is acidic and can donate a proton (), becoming a negatively charged carboxylate ion ().
- The amine group () is basic and can accept a proton, becoming a positively charged ammonium group (). Due to this dual nature, amino acids are amphoteric, meaning they can react with both acids and bases.
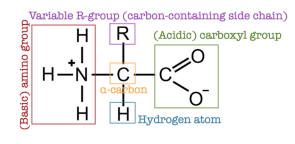
Zwitterions and Isoelectric Point
In aqueous solutions, amino acids typically exist in a form called a zwitterion. A zwitterion is a molecule that has both positive and negative charges but is overall electrically neutral:
- In a zwitterionic form, the amino acid has a deprotonated carboxyl group () and a protonated amine group ().
- The specific pH at which an amino acid exists predominantly in its zwitterionic form is called the isoelectric point (pI). At this point, the amino acid has no net charge, as the positive and negative charges balance each other out. Amino acids can exist in molecular form, in cationic form, in anionic form or in Zwitterion form depending on the environment:
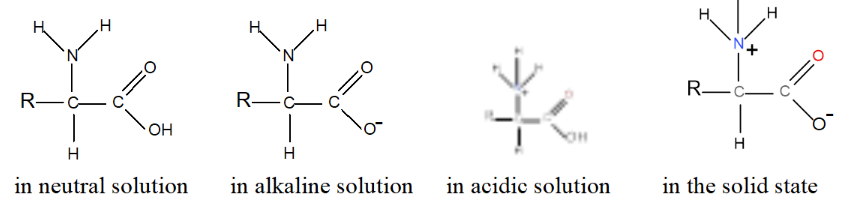
Since amino acids can react with acids and alkalis, they make very effective buffer solutions.
Reaction with Bases
When an amino acid reacts with a base (e.g., sodium hydroxide, ):
- The base can cause the amino acid's carboxyl group to lose a proton, increasing its negative charge and forming a carboxylate ion ().
- This reaction highlights the acidic nature of the carboxylic acid group in amino acids, as it donates a proton to the basic solution. Understanding the structure and behavior of amino acids, especially their ability to form zwitterions, is essential in grasping their roles in biological systems and their reactivity in different pH environments.
General Structure
General structure of an amino acid involves one 'central' C atom with bonds to 4 groups: • Hydrogen atom • Amino group • Carboxyl group • Organic 'side' group, basically a variable length chain
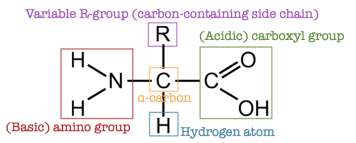
These amino acids are called α-amino acids or 2-amino acids. • All 20 naturally occurring amino acids are α-amino acids, they differ only in their R-groups. • All 20 amino acids are optically active except for one, 2-amino ethanoic acid (where R-group is hydrogen atom). • In nature, almost all amino acids exist as one enantiomer only.
Reactions due to Functional Groups
• The amino group reacts as an amine, so it can be:
- Protonated by acids. - Acylated with acyl chloride or acid anhydride. - Nucleophilic substitution with halogenoalkanes. • The carboxyl group reacts as a carboxylic acid, so it can be:
- De-protonated by bases. - Esterified with alcohols (if there's an acid catalyst present).
Acid base properties
Amino acids have acidic and basic properties, they're amphoteric: • The carboxylic acid group has a tendency to lose a proton (act as an acid):
- COOH ⇌ -COO- + H+
- The amine group has a tendency to gain a proton (act as a base):
- NH2 + H+ ⇌ -NH3+
Zwitterion (zuh-vit-air-eye-on)
• If we take the general case of an amino acid, at a pH close to neutral, the carboxylic acid group will lose a proton AND the amine group will gain a proton (as shown below). •The resulting species is a dipolar ion which has no net charge •This is called a zwitterion. •SOMETIMES, AQA will try to catch you out by having more than 1 acid and 1 amino group and then ask you: which one of these is a zwitterion •The answer is the one with no net charge
The ionic nature of amino acids explains why they have the following physical properties: • High m.p. colourless solids at room temperature
- Solid amino acids contain ionic bonds, which leads to the higher than expected m.p. • Soluble in water but not in non-polar solvents. • Weakly acidic and weakly basic.
Amino Acids at Low + High pH Levels
• In an acidic (low pH) environment, there will be a high conc. of H+ • So, the amine group will gain a proton - it's protonated. • In a basic (high pH) environment, there will be a low conc. of H+. • So, the carboxylic acid group will lose a proton - it's deprotonated.
500K+ Students Use These Powerful Tools to Master Amino Acids For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
49 flashcards
Flashcards on Amino Acids
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards5 quizzes
Quizzes on Amino Acids
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Amino Acids
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Amino Acids
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Amino Acids
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Amino Acids you should explore
Discover More Revision Notes Related to Amino Acids to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Amino acids, Proteins & DNA (A Level only)
Anticancer Drugs
370+ studying
186KViews