Photo AI
Last Updated Sep 27, 2025
Organic Synthesis Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Organic Synthesis quickly and effectively.
342+ students studying
7.8.1 Organic Synthesis
Organic synthesis involves creating complex molecules from simpler substances through a series of reactions. A well-designed synthetic route is crucial for efficiency, sustainability, and safety. This guide focuses on key aims in synthesis, the rationale behind them, and illustrative reaction sequences.
- The synthesis of an organic compound can involve several steps.
- Chemists use synthetic routes to show the reagents, conditions, and any special procedures needed to get from one compound to another.
Key Aims in Organic Synthesis Design
- Avoiding Solvents and Hazardous Starting Materials
- Rationale: Using solvents can present environmental and health risks. By minimizing or eliminating solvents, chemists reduce waste, lower costs, and avoid potential hazards associated with solvent recovery or disposal.
- Green Chemistry: Choosing starting materials that are non-toxic and biodegradable aligns with green chemistry principles, reducing harmful emissions and improving safety.
- Minimizing Reaction Steps with High Atom Economy
- Rationale: A synthesis with fewer steps not only saves time but also reduces the likelihood of side reactions and degradation, resulting in a higher yield of the desired product.
- Atom Economy: This metric evaluates the efficiency of a reaction based on the proportion of reactants that become part of the final product. Higher atom economy is preferred because it indicates less waste and more sustainable reactions.
Devising a Step-Wise Synthesis for an Organic Compound
In your exam you could be asked to devise a step-wise synthesis, with up to 4 steps.
These are all for organic compounds. When answering the question, make sure to include:
- Special procedures (e.g. refluxing).
- Conditions (e.g. high temp./pressure or catalyst).
- Safety precautions (e.g. fume cupboard for toxic gases such as HCl or HCN; avoid splashes on bare skin for bromine water, acids or alkalis).
Example Synthetic Routes (up to Four Steps): Here are some example pathways for synthesizing organic compounds:
- Synthesis of Ethanoic Acid from Ethanol:
- Step 1: Oxidize ethanol (CH₃CH₂OH) with acidified potassium dichromate (K₂Cr₂O₇/H⁺) to form ethanal (CH₃CHO).
- Step 2: Reflux ethanal with further potassium dichromate to oxidize it fully to ethanoic acid (CH₃COOH).
- Conversion of Ethene to Ethanol:
- Step 1: Hydrate ethene (CH₂=CH₂) by reacting it with steam in the presence of phosphoric acid (H₃PO₄) as a catalyst to produce ethanol (CH₃CH₂OH).
- Preparation of Bromoethane from Ethene:
- Step 1: Add hydrogen bromide (HBr) to ethene, resulting in bromoethane (CH₃CH₂Br) via electrophilic addition.
- Synthesis of Ethyl Ethanoate (an Ester):
- Step 1: React ethanol (CH₃CH₂OH) with ethanoic acid (CH₃COOH) in the presence of sulfuric acid (H₂SO₄) as a catalyst to produce the ester ethyl ethanoate (CH₃COOCH₂CH₃) and water.
Designing Synthetic Routes
When designing synthetic routes, chemists aim to:
- Design processes that do not require a solvent. - Avoiding solvents reduces the hazards associated with the process and the amount of waste created from a synthetic route.
- Solvents are often flammable and toxic, so pose safety risks.
- Having to separate solvents from products or dispose of solvents after the reaction is complete can create a lot of waste too.
- Design processes that use non-hazardous starting materials. - These may be toxic, corrosive, or flammable.
- Often the use of KCN is avoided as it is toxic, and other synthetic routes will be used instead - This limits the potential for accidents and environmental damage.
- Design production methods with fewer steps that have a high % atom economy.
- Chemists will always try to limit the number of steps in a conversion as there is a loss at each step as the % yield is not 100% for each reaction. - A higher % atom economy means more of the starting materials are converted into useful products, which minimises waste.
Types of Reactions Used in Organic Synthesis
A variety of reactions are used to construct organic molecules, including:
- Reduction Reactions: These involve adding a reducing agent to the molecule, effectively adding hydrogen or removing oxygen. Reduction can convert carbonyl compounds into alcohols, a useful transformation in many synthetic pathways.
- Oxidation Reactions: Oxidation generally adds oxygen or removes hydrogen. For example, primary alcohols can be oxidized to aldehydes, and further oxidation can produce carboxylic acids. Common oxidizing agents include acidified potassium dichromate (K₂Cr₂O₇).
- Hydrolysis: This reaction involves breaking down compounds by adding water, often splitting esters into an alcohol and a carboxylic acid, or amides into amines and carboxylic acids.
- Substitution Reactions: In substitution, one atom or group of atoms in a molecule is replaced with another. For example, halogenoalkanes can undergo nucleophilic substitution with ammonia to form amines.
- Addition Reactions: Addition reactions occur when two or more molecules combine to form a single product. This is common in alkenes, where the double bond opens up, allowing new atoms or groups to add across the bond.
- Condensation Reactions: Two molecules join to form a larger molecule, often releasing a small molecule such as water in the process. An example is esterification, where an alcohol and a carboxylic acid combine to form an ester and water.
Practical Applications: Common Examples in Organic Synthesis
- Wittig Reaction: Used to form carbon-carbon double bonds, this reaction uses a phosphorus ylide to convert aldehydes or ketones into alkenes. This is an important method for forming alkenes in organic synthesis.
- Grignard Reaction: This organometallic reaction adds a Grignard reagent (e.g., alkyl-magnesium halides) to carbonyl compounds (like aldehydes or ketones) to form alcohols. This reaction is highly valuable for building carbon skeletons in organic molecules.
- Use of Catalysts in Organic Synthesis
- Role of Catalysts: Catalysts are substances that increase the reaction rate without being consumed in the reaction. They offer an alternative pathway with a lower activation energy, allowing reactions to proceed faster with less energy input.
- Types of Catalysts: Catalysts are categorized as homogeneous (soluble in the reaction mixture) or heterogeneous (insoluble and provide a surface for the reaction). Enzymes, as highly specific biological catalysts, are increasingly used for their efficiency and mild operating conditions.
- Industrial Impact: Catalysts make reactions more efficient, selective, and faster, which is especially valuable for large-scale production of pharmaceuticals, plastics, and other commercial products.
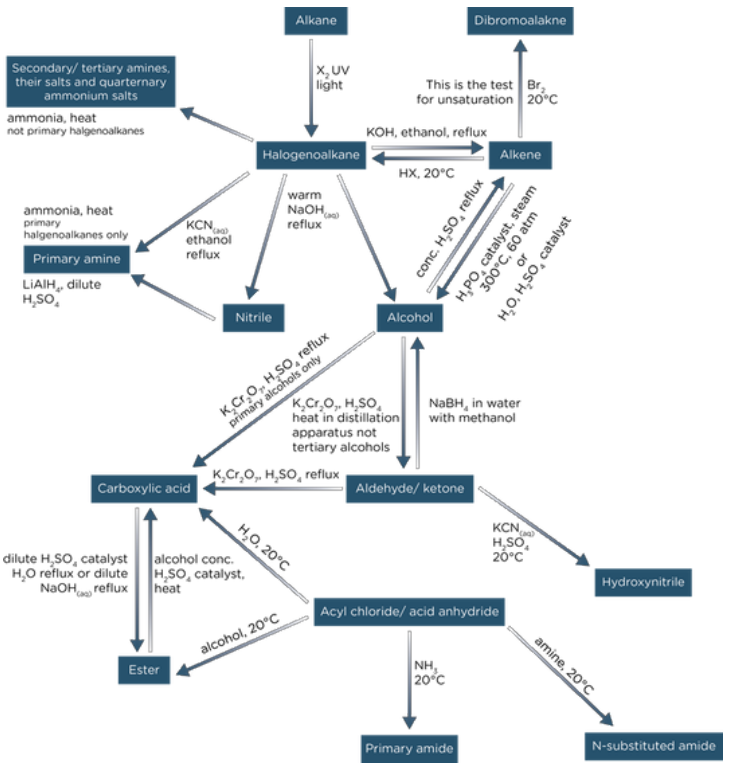
Exam Technique:
- In the exam you may be asked to 'identify' the name of a reagent used in a synthesis step.
- A reagent is just a chemical that can be used straight out of a bottle or container, for example, HCl or KCN. E.g. Suggest the reagents used to make a halogenoalkane into a nitrile. KCN and ethanol.
500K+ Students Use These Powerful Tools to Master Organic Synthesis For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
10 flashcards
Flashcards on Organic Synthesis
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards1 quizzes
Quizzes on Organic Synthesis
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Organic Synthesis
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Organic Synthesis
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Organic Synthesis
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Organic Synthesis you should explore
Discover More Revision Notes Related to Organic Synthesis to Deepen Your Understanding and Improve Your Mastery
Load more notes