Photo AI
Last Updated Sep 27, 2025
Organic Mechanisms Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Organic Mechanisms quickly and effectively.
216+ students studying
7.9.1 Organic Mechanisms
Mechanisms in Organic Chemistry
In organic chemistry, a reaction mechanism explains the sequence of steps by which reactants are transformed into products. These mechanisms provide a detailed, step-by-step description of how bonds break and form during a reaction. Understanding mechanisms is crucial for predicting the outcomes of reactions, optimizing reaction conditions, and designing new synthetic methods.
Curly Arrows and Electron Movement
Curly arrows are used in organic mechanisms to represent the movement of electron pairs:

-
Start of the Arrow: The arrow begins at the original position of the electron pair (either a lone pair or a bond).
-
End of the Arrow: The arrow points towards where the electron pair is going, usually to form a new bond or to break an existing bond. Curly arrows are drawn with specific conventions:
-
Half Curly Arrows represent the movement of a single electron (used in free radical mechanisms).
-
Full Curly Arrows represent the movement of an electron pair.
Types of Bond Breaking in Mechanisms
- Homolytic Fission: In homolytic fission, the bond breaks evenly, and each atom receives one of the electrons from the covalent bond.
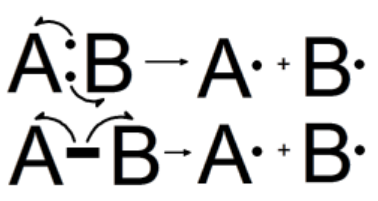
- This type of fission produces free radicals, which are highly reactive species with an unpaired electron.
- A half curly arrow is used to indicate the movement of one electron to each atom.
- Heterolytic Fission:
-
In heterolytic fission, the bond breaks unevenly, with both electrons moving to one atom (typically the more electronegative atom).
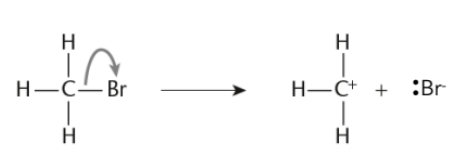
-
This results in the formation of ions: one atom receives the electron pair and becomes negatively charged, while the other becomes positively charged.
-
A full curly arrow is used to show the movement of the electron pair.
Bond Making and Curly Arrows
When forming a bond, the curly arrow starts at a lone pair (or an existing bond) and points towards the atom with which the new bond is being formed. This shows the donation of an electron pair to create a covalent bond between atoms.
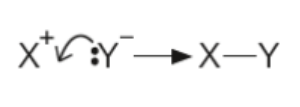
Guidelines for Drawing Mechanisms
To draw accurate mechanisms, follow these pointers:
- Displayed Formula: Draw the full displayed structure for each molecule involved, including all bonds and lone pairs.
- Curly Arrows: Ensure that curly arrows start from a lone pair or bond and point to the destination atom.
- Balance Charges: Make sure that charges on each side of the reaction are balanced; if the reactants have a net charge, the products should have the same net charge.
- Exclude Spectator Ions: Only include species involved in the reaction; spectator ions that do not change should be omitted.
Types of Reaction Mechanisms
- Substitution Reactions:
- A substitution reaction involves replacing one atom or group with another.
- Example: Halogenoalkanes undergoing nucleophilic substitution with a nucleophile (e.g., or ) to replace the halogen.
- Addition Reactions:
- In an addition reaction, two reactants combine to form a single product.
- Example: The addition of hydrogen to an alkene to form an alkane (hydrogenation).
- Elimination Reactions:
- Elimination involves the removal of a small molecule from a larger molecule, typically forming a double bond.
- Example: Dehydration of alcohols to form alkenes and water.
- Oxidation and Reduction:
- Oxidation is an increase in the oxygen content or a decrease in hydrogen content.
- Reduction is a decrease in oxygen content or an increase in hydrogen content.
- Example: Oxidation of primary alcohols to form aldehydes, then further oxidation to carboxylic acids.
- Hydrolysis:
- Hydrolysis is the breakdown of a molecule by water.
- Example: The hydrolysis of esters in the presence of an acid or alkali to form a carboxylic acid and alcohol.
Free Radical Mechanisms
Free radicals are atoms or molecules with an unpaired electron, formed by homolytic fission. Free radical mechanisms are chain reactions that proceed through three stages:
- Initiation:
- Free radicals are generated by homolytic fission, often triggered by UV light.
- Example: In the chlorination of methane, chlorine molecules split to form two chlorine radicals. Here is the chemical reaction from the image written out as a linear equation:
- Propagation:
- Free radicals react with stable molecules to form new radicals, propagating the reaction chain.
- Example: Chlorine radicals react with methane to form methyl radicals and , which then react further to produce more chlorine radicals and methyl chloride.
The chlorine radical is regenerated in the second step, allowing the reaction to continue as a chain reaction.
- Termination:
- The reaction terminates when two free radicals react to form a stable molecule, ending the chain reaction.
- Example: Methyl radicals combine to form ethane, or chlorine radicals combine to reform . Here are the three chemical equations from the image written as linear equations:
These are termination steps in a free radical mechanism, where the radicals combine to form stable molecules, ending the chain reaction.
Example: Depletion of Ozone
A classic example of a free radical mechanism is the depletion of ozone in the atmosphere. Chlorine radicals, often produced by CFCs (chlorofluorocarbons), catalyze the breakdown of ozone:
- Initiation: Chlorine radicals () are formed when CFCs break down under UV light. Here is the chemical reaction from the image written out as a linear equation:
- Propagation: Chlorine radicals react with ozone () to form and , then· reacts with another molecule to regenerate the radical and produce more . Here are the two chemical equations from the image written out as linear equations:
- Termination: The chain continues until two radicals (such as two Cl· radicals) meet and combine, stopping the reaction.
The loss of ozone is a significant environmental issue because the ozone layer protects Earth from harmful UV radiation.
Importance of Understanding Mechanisms
Mastering mechanisms allows chemists to:
- Predict products and optimize reaction conditions.
- Identify the rate-determining step and make modifications to improve yield.
- Design new synthetic pathways for the creation of desired compounds.
500K+ Students Use These Powerful Tools to Master Organic Mechanisms For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
10 flashcards
Flashcards on Organic Mechanisms
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards1 quizzes
Quizzes on Organic Mechanisms
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Organic Mechanisms
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Organic Mechanisms
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Organic Mechanisms
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Organic Mechanisms you should explore
Discover More Revision Notes Related to Organic Mechanisms to Deepen Your Understanding and Improve Your Mastery
Load more notes