Photo AI
Last Updated Sep 27, 2025
Colorimetry & Complex Ions Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Colorimetry & Complex Ions quickly and effectively.
250+ students studying
8.2.6 Colorimetry & Complex Ions
Aim:
To determine the concentration of a coloured solution by measuring the absorbance of light using a colorimeter, and to apply this technique to metal aqua ions. This practical will involve preparing a series of solutions of known concentrations, measuring their absorbances, and plotting a calibration curve to find the concentration of an unknown solution.
Introduction:
Colorimetry is a technique that measures the absorption of light by a coloured solution. The amount of light absorbed is proportional to the concentration of the solution. This relationship allows us to determine the concentration of an unknown solution by measuring its absorbance and comparing it to a calibration curve made from known concentrations.
When light passes through a coloured solution, certain wavelengths are absorbed while others are transmitted. The complementary colour of the solution is the colour that is transmitted. For example, a hexaaquacopper(II) solution appears blue because it absorbs red light.
Principle of Colorimetry:
- Greater concentration of a solution = greater absorbance of light.
- A calibration curve is used to compare the absorbance of known concentrations to the absorbance of an unknown concentration.
- A colour filter is used to allow only the complementary colour to pass through, ensuring the light absorbed is specific to the colour of the solution. For example, a pale blue hexaaquacopper(II) solution transmits blue light and absorbs red light, so a red filter would be used in the colorimeter.
Materials and Equipment:
- Metal aqua ion solution (e.g., hexaaquacopper(II))
- Standard solutions of known concentrations
- Distilled water (for dilution)
- Colorimeter
- Coloured filter (specific to the complementary colour of the solution)
- Cuvettes
- Volumetric flask and pipette (for preparing solutions)
- Graph paper or plotting software (for calibration curve)
- Burette and beakers
Method:
Step 1: Prepare Standard Solutions
- Prepare a range of metal aqua ion solutions of known concentrations by diluting a stock solution using distilled water. For example:
- Prepare concentrations such as 0.10 mol dm⁻³, 0.08 mol dm⁻³, 0.06 mol dm⁻³, 0.04 mol dm⁻³, and 0.02 mol dm⁻³.
- Use a volumetric flask and pipette to ensure accurate measurement of each solution.
Step 2: Set Up the Colorimeter
- Select the appropriate colour filter based on the complementary colour of your metal aqua ion solution. For a blue solution, use a red filter, since the solution absorbs red light.
- Calibrate the colorimeter by placing a cuvette filled with distilled water (the blank) into the colorimeter and setting the absorbance to zero.
Step 3: Measure Absorbances of Known Concentrations
- Rinse a cuvette with a small amount of the first standard solution (0.10 mol dm⁻³), then fill the cuvette about ¾ full with the solution.
- Place the cuvette in the colorimeter and record the absorbance.
- Repeat for each concentration of the standard solutions, rinsing the cuvette between each measurement.
- Record the absorbance for each concentration.
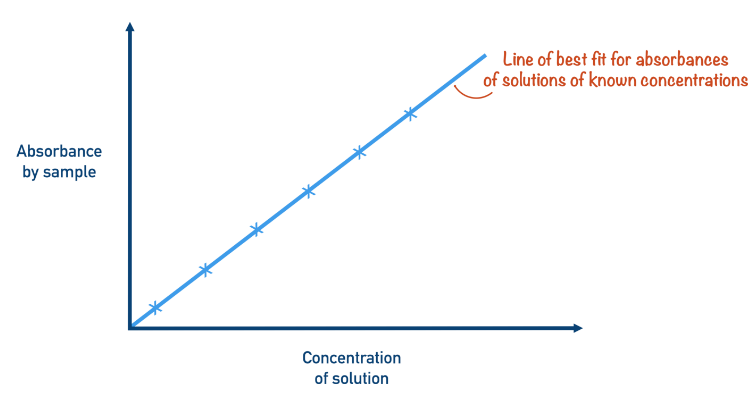
Step 4: Plot a Calibration Curve
- Using the recorded absorbances and corresponding concentrations, plot a calibration curve with:
- Concentration on the x-axis
- Absorbance on the y-axis
- Draw a line of best fit through the data points. This line can be used to determine the concentration of unknown solutions by interpolation.
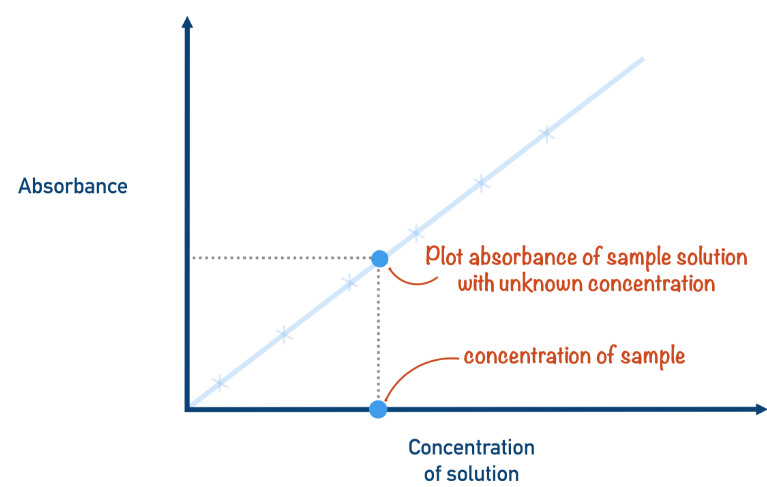
Step 5: Measure the Absorbance of the Unknown Solution
- Rinse the cuvette with a sample of the unknown concentration solution, then measure its absorbance using the colorimeter.
- Locate the absorbance value of the unknown solution on the y-axis of your calibration curve. Draw a line across to intersect the calibration line, then draw a vertical line down to the x-axis to find the concentration of the unknown solution.
Results and Analysis:
| Concentration (mol dm⁻³) | Absorbance |
|---|---|
| 0.10 | 0.85 |
| 0.08 | 0.70 |
| 0.06 | 0.55 |
| 0.04 | 0.40 |
| 0.02 | 0.25 |
| Unknown | 0.65 |
- From the calibration curve, locate the absorbance of 0.65. The corresponding concentration might be around 0.075 mol dm⁻³.
- Calculation of Concentration: Using the calibration curve, the concentration of the unknown solution can be determined accurately.
Worked Example:
Question: Outline a practical method for determining the concentration of an unknown metal aqua ion solution using colorimetry.
Answer:
- Prepare a series of standard solutions with known concentrations of the metal aqua ion.
- Set up the colourimeter with the appropriate complementary colour filter.
- Measure the absorbance of each standard solution and plot a calibration curve of absorbance versus concentration.
- Measure the absorbance of the unknown solution.
- Use the calibration curve to determine the concentration of the unknown solution by comparing its absorbance to that of the known concentrations.
Important Notes:
- Complementary Colour: The colour filter should allow only the complementary colour to pass through the solution. For example, a blue solution absorbs red light, so use a red filter.
- Calibration Curve: Always plot a calibration curve using several concentrations to ensure accuracy. Ensure that your line of best fit is smooth and passes through as many points as possible.
500K+ Students Use These Powerful Tools to Master Colorimetry & Complex Ions For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
90 flashcards
Flashcards on Colorimetry & Complex Ions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards9 quizzes
Quizzes on Colorimetry & Complex Ions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Colorimetry & Complex Ions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Colorimetry & Complex Ions
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Colorimetry & Complex Ions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Colorimetry & Complex Ions you should explore
Discover More Revision Notes Related to Colorimetry & Complex Ions to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Inorganic Chemistry Practicals (A Level only)
Required Practical 11
332+ studying
186KViews96%
114 rated
Inorganic Chemistry Practicals (A Level only)
Testing Period 3 Oxides
425+ studying
187KViews96%
114 rated
Inorganic Chemistry Practicals (A Level only)
Ligand Substitution Experiments
241+ studying
200KViews96%
114 rated
Inorganic Chemistry Practicals (A Level only)
Redox Titrations
386+ studying
195KViews