Photo AI
Last Updated Sep 27, 2025
Definitions Simplified Revision Notes for A-Level AQA Physics
Revision notes with simplified explanations to understand Definitions quickly and effectively.
421+ students studying
Definitions
Alpha Decay
- Definition: Alpha decay is a type of radioactive decay where an unstable nucleus releases an alpha particle, which consists of two protons and two neutrons. This process reduces the mass and atomic number of the original nucleus, making it more stable.
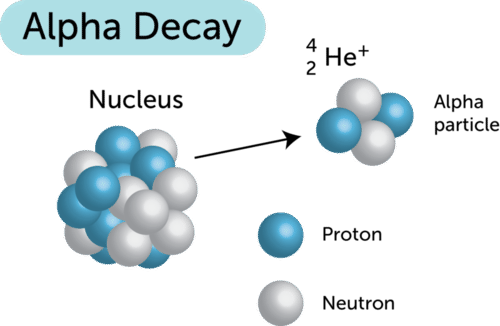
- Example: When Uranium-238 undergoes alpha decay, it emits an alpha particle and transforms into Thorium-234.
Annihilation
- Definition: Annihilation occurs when a particle collides with its antiparticle. This collision converts their combined mass into energy, typically released as two gamma photons. The energy released is in line with Einstein's E = mc² principle.
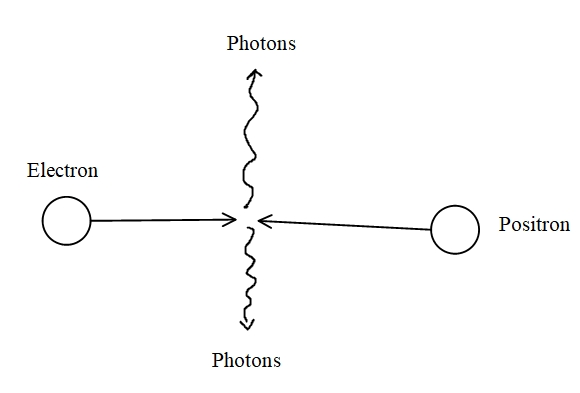
- Explanation: Conservation of momentum is ensured by the emission of two photons in opposite directions.
Antiparticle
- Definition: Every particle has a corresponding antiparticle that shares the same mass but has opposite charge and other opposite properties, such as baryon and lepton numbers.
- Examples: The antiparticle of an electron is the positron, while the antiproton is the counterpart of a proton.
Baryon Number
- Definition: A conserved quantum number representing the number of baryons (heavy particles) in a system. Baryons have a baryon number of +1, while antibaryons have -1. This number remains unchanged in particle interactions.
- Example: In any particle reaction, the total baryon number before and after the reaction must be the same.
Baryons
- Definition: Baryons are a group of hadrons made up of three quarks. The proton is the only stable baryon, while the neutron, also a baryon, is stable only within a nucleus.
- Key Point: Baryons are subject to the strong nuclear force and decay via the weak interaction if unstable.
Beta Decay
- Beta-Minus Decay: A neutron in a nucleus converts into a proton, emitting an electron (beta particle) and an antineutrino.
- Beta-Plus Decay: A proton converts into a neutron, emitting a positron and a neutrino. This type of decay is common in proton-rich nuclei.
Electron Diffraction
- Definition: When electrons pass through a small gap comparable to their de Broglie wavelength, they exhibit wave-like properties, spreading out in a pattern.
- Significance: Electron diffraction is evidence of wave-particle duality in electrons, supporting the idea that particles can behave as waves under certain conditions.
Electron Volt (eV)
- Definition: An electron volt (eV) is the energy gained by an electron when it is accelerated through a potential difference of 1 volt. 1 eV is equal to 1.6 × 10⁻¹⁹ joules.
- Application: The electron volt is a convenient unit of energy, particularly in atomic and nuclear physics, due to the small energies involved.
Energy Levels
- Definition: Electrons within an atom exist at specific energy levels or shells. Electrons cannot exist in-between these levels.
- Explanation: When an electron absorbs or releases energy, it jumps between these levels, often emitting light as a photon when returning to a lower energy state.
Excitation
- Definition: Excitation occurs when an electron absorbs exactly the right amount of energy to move to a higher energy level within an atom.
- Example: Excited electrons return to lower levels by releasing energy as light, which forms the basis of atomic spectra.
Gauge Boson
- Definition: Gauge bosons are particles that mediate the four fundamental forces. Each force has its corresponding boson: photons for electromagnetism, W and Z bosons for the weak force, gluons for the strong force, and gravitons (hypothetical) for gravity.
- Key Point: Gauge bosons are responsible for the interactions between particles, enabling forces to act over distances.
Ground State
- Definition: The ground state is the lowest energy level that an electron can occupy in an atom. It is the most stable state.
- Importance: Electrons in higher energy levels naturally return to the ground state, releasing energy as they do so.
Hadrons
- Definition: Hadrons are particles that are subject to the strong nuclear force and are composed of quarks. There are two main categories:
- Baryons (three quarks), such as protons and neutrons.
- Mesons (quark-antiquark pairs), like pions and kaons.
Ionisation
- Definition: Ionisation is the process where an atom loses or gains an electron, becoming a charged ion.
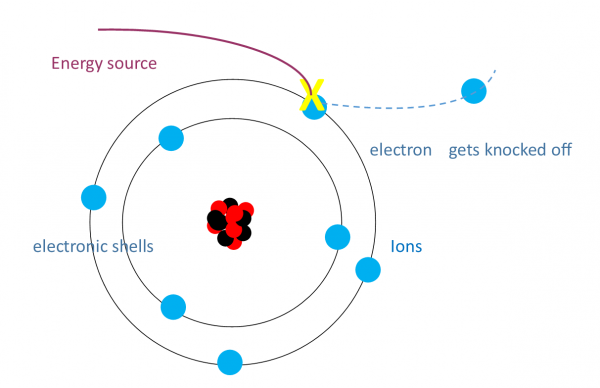
- Example: When energy sufficient to overcome the ionisation energy is supplied, an electron is ejected, leaving a positively charged ion.
Isotope
- Definition: Isotopes are atoms of the same element with the same proton number but different neutron numbers.
- Example: Carbon-12 and Carbon-14 are isotopes of carbon, with different neutron counts but identical chemical properties.
Pair Production
- Definition: Pair production occurs when a high-energy photon is converted into a particle-antiparticle pair (such as an electron and a positron) near a nucleus.
- Explanation: The process requires high photon energy and usually occurs near a nucleus to conserve momentum.
Photon
- Definition: A photon is a quantum of electromagnetic radiation and acts as a particle of light.
- Energy: Given by E = hf, where h is Planck's constant and f is the frequency of the radiation.
Strange Particles and Strangeness
- Definition: Strange particles are created through the strong interaction but decay via the weak interaction.
- Strangeness: A quantum number conserved in strong interactions but can change in weak interactions, reflecting that strange particles are typically produced in pairs.
Strong Nuclear Force
- Definition: This is the fundamental force that binds protons and neutrons within the nucleus, overcoming the electromagnetic repulsion between protons.
- Range: It acts attractively up to about 3 femtometres (fm) and becomes repulsive below 0.5 fm to prevent the collapse of nucleons.
Threshold Frequency
- Definition: The minimum frequency of light required to release electrons from a metal's surface through the photoelectric effect.
- Relation to Work Function: Threshold frequency is calculated as f_min = φ/h, where φ is the work function, and h is Planck's constant.
Work Function
- Definition: The work function is the minimum energy needed to release an electron from a metal surface.
- Application: In the photoelectric effect, only photons with energy equal to or greater than the work function can eject electrons.
500K+ Students Use These Powerful Tools to Master Definitions For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
10 flashcards
Flashcards on Definitions
Revise key concepts with interactive flashcards.
Try Physics Flashcards1 quizzes
Quizzes on Definitions
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes29 questions
Exam questions on Definitions
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Definitions
Create custom exams across topics for better practice!
Try Physics exam builder56 papers
Past Papers on Definitions
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Definitions you should explore
Discover More Revision Notes Related to Definitions to Deepen Your Understanding and Improve Your Mastery
Load more notes