Photo AI
Last Updated Sep 27, 2025
Coulomb’s law Simplified Revision Notes for A-Level AQA Physics
Revision notes with simplified explanations to understand Coulomb’s law quickly and effectively.
376+ students studying
7.3.1 Coulomb's law
Definition
Coulomb's Law states that the force between two point charges in a vacuum is:
- Directly proportional to the product of the two charges.
- Inversely proportional to the square of the distance between them. The mathematical expression for Coulomb's law is:
Where:
- F is the force between the charges,
- is the permittivity of free space,
- and are the charges,
- is the distance between the centres of the charges.
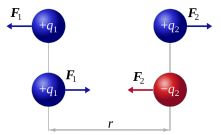
Important Notes:
-
Air can be approximated as a vacuum for the purposes of calculations with Coulomb's law.
-
For a spherical charge distribution, the charge can be considered to act from the centre of the sphere. Force Direction:
-
If both charges are of the same sign, the force is repulsive.
-
If the charges have opposite signs, the force is attractive.
Comparing Electrostatic and Gravitational Forces
The electrostatic force between subatomic particles (like protons and electrons) is generally much stronger than the gravitational force acting between them. This difference is due to the relatively small masses and larger charges of these particles. Here's a calculation example for illustration:
Consider two protons, 2 pm (or 2 × 10⁻¹² m) apart.
- Gravitational Force Calculation:
- Electrostatic Force Calculation:
- Ratio of Electrostatic Force to Gravitational Force:
Conclusion:
The electrostatic force between two protons is approximately 1.24 × 10³⁶ times stronger than the gravitational force. This significant difference is why electrostatic forces dominate interactions at the atomic and subatomic levels.
500K+ Students Use These Powerful Tools to Master Coulomb’s law For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
30 flashcards
Flashcards on Coulomb’s law
Revise key concepts with interactive flashcards.
Try Physics Flashcards3 quizzes
Quizzes on Coulomb’s law
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes29 questions
Exam questions on Coulomb’s law
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Coulomb’s law
Create custom exams across topics for better practice!
Try Physics exam builder56 papers
Past Papers on Coulomb’s law
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Coulomb’s law you should explore
Discover More Revision Notes Related to Coulomb’s law to Deepen Your Understanding and Improve Your Mastery
