Photo AI
Last Updated Sep 26, 2025
Electronic Structure Simplified Revision Notes for GCSE AQA Chemistry Combined Science
Revision notes with simplified explanations to understand Electronic Structure quickly and effectively.
255+ students studying
1.1.10 Electronic Structure
Energy Levels:
- Electrons in an atom occupy energy levels. These are also called shells or orbits. Each electron in an atom is found in a particular energy level.
- The lowest energy level (innermost shells) fills with electrons first.
- Each energy level can only hold a certain number of electrons before it becomes full.
- The first energy level can hold a maximum of two electrons, the second energy level a maximum of eight, and so on.
Electrons in the first three energy levels for elements with atomic numbers 1 to 20 are distributed as follows:
| Energy level or shell | Maximum number of electrons |
|---|---|
| First | 2 |
| Second | 8 |
| Third | 8 |
Important Note: You need to be able to write the electronic structure of any of the first twenty elements.
Writing an Electronic Structure
The electronic structure of an atom is written using numbers to represent the electrons in each energy level.
For example, for sodium, this is 2,8,1 – showing that there are:
- 2 electrons in the first energy level.
- 8 electrons in the second energy level.
- 1 electron in the third energy level.
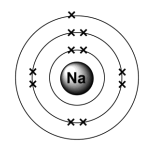
You can work out the electronic structure of an atom from its atomic number or its position in the periodic table.
- Start at hydrogen (H), and count the elements needed to reach the element you are interested in.
- For sodium, it takes:
- 2 elements to reach the end of the first period (row).
- 8 elements to reach the end of the second period.
- 1 element to reach sodium in the third period.
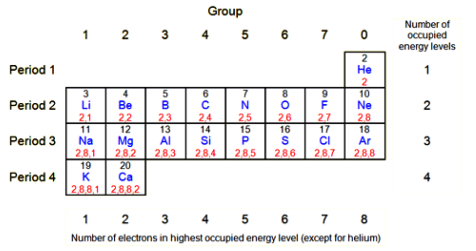
The diagram of the periodic table shows how this works, with the number of electrons in the highest occupied energy level for each element.
500K+ Students Use These Powerful Tools to Master Electronic Structure For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
110 flashcards
Flashcards on Electronic Structure
Revise key concepts with interactive flashcards.
Try Chemistry Combined Science Flashcards11 quizzes
Quizzes on Electronic Structure
Test your knowledge with fun and engaging quizzes.
Try Chemistry Combined Science Quizzes29 questions
Exam questions on Electronic Structure
Boost your confidence with real exam questions.
Try Chemistry Combined Science Questions27 exams created
Exam Builder on Electronic Structure
Create custom exams across topics for better practice!
Try Chemistry Combined Science exam builder25 papers
Past Papers on Electronic Structure
Practice past papers to reinforce exam experience.
Try Chemistry Combined Science Past PapersOther Revision Notes related to Electronic Structure you should explore
Discover More Revision Notes Related to Electronic Structure to Deepen Your Understanding and Improve Your Mastery
