Photo AI
Last Updated Sep 26, 2025
States of Matter Simplified Revision Notes for GCSE AQA Chemistry Combined Science
Revision notes with simplified explanations to understand States of Matter quickly and effectively.
269+ students studying
2.2.1 States of Matter
Solids, Liquids and Gases
The arrangement and movement of particles vary depending on whether the substance is in a solid, liquid, or gas state. The table below compares these three states of matter:
Summary:
- Solids have a fixed shape, cannot flow, and are incompressible due to tightly packed particles.
- Liquids can flow and take the shape of their container but are also incompressible because their particles are close together.
- Gases flow, expand to fill their container, and are compressible because their particles are far apart and move rapidly in all directions.
| State | Closeness of Particles | Arrangement of Particles | Movement of Particles | Energy of Particles |
|---|---|---|---|---|
| Solid | Very close | Regular pattern | Vibrate around a fixed position | Low energy |
| Liquid | Close | Randomly arranged | Move around each other | Greater energy |
| Gas | Far apart | Randomly arranged | Move quickly in all directions | Highest energy |
The particles in these diagrams could represent atoms, molecules, or ions, depending on the type of substance (e.g., ionic compounds, small molecules, giant molecules, or metals).
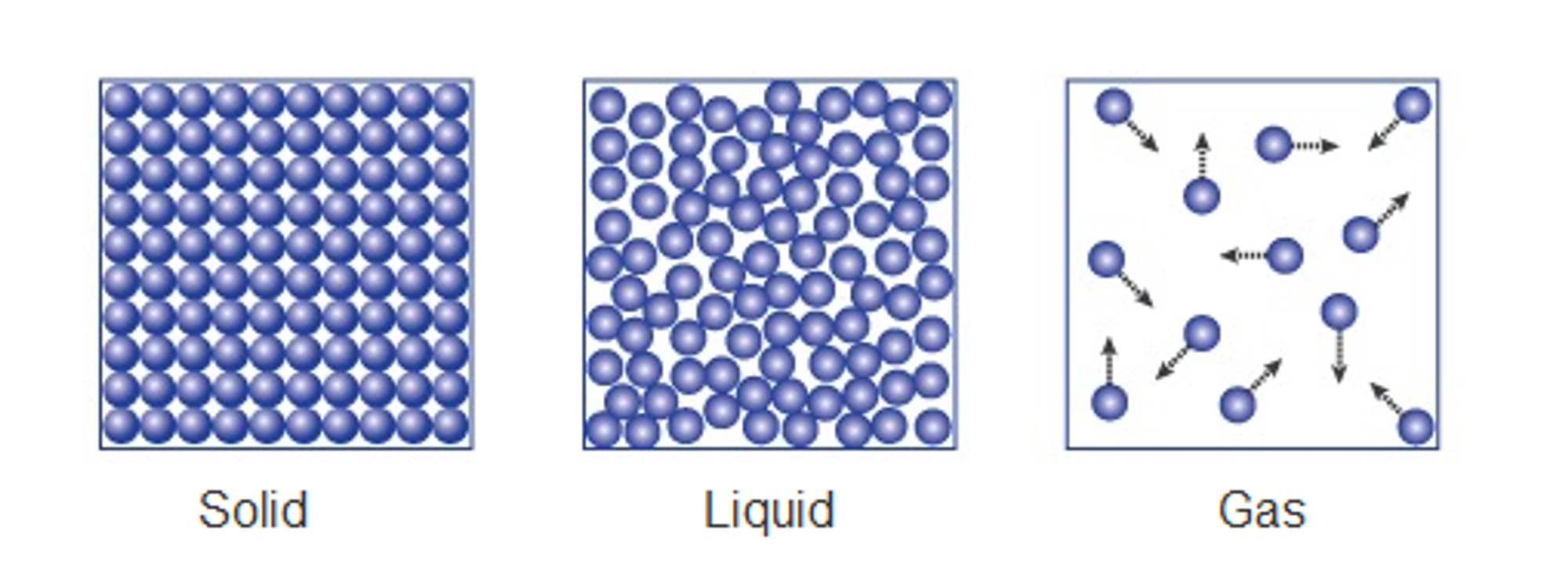
Solids:
- Fixed Shape and No Flow:
- Particles in a solid are closely packed in a regular pattern and can only vibrate around fixed positions. This restricts their movement, preventing the solid from flowing.
- Incompressibility:
- Because the particles are so close together, there is no space for them to move closer, making solids difficult to compress.
Liquids:
- Flow and Shape:
- Particles in a liquid are close together but arranged randomly and can move around each other. This allows the liquid to flow and take the shape of its container.
- Incompressibility:
- Similar to solids, the particles in a liquid are close together, leaving little space for compression.
Gases:
- Flow and Expansion:
- Particles in a gas are far apart and move quickly in all directions. This allows gases to flow freely and completely fill any container they occupy.
- Compressibility:
- The large spaces between particles in a gas mean that the particles can be pushed closer together, making gases easily compressible.
Changes of state
The diagram illustrates the common changes of state between solids, liquids, and gases.
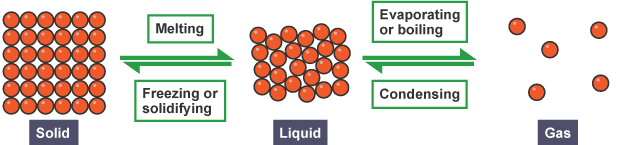
Sublimation: Some substances can change directly from a solid to a gas without becoming a liquid in between. This process is called sublimation. An example of this is solid carbon dioxide, commonly known as 'dry ice', which can sublime directly into a gas.
Melting, Evaporating, and Boiling
Energy Transfer: To change the state of a substance from solid to liquid (melting) or liquid to gas (evaporating or boiling), energy must be transferred to the substance by heating.
Particle Behavior:
- During melting, energy is used to break some of the bonds between particles.
- During evaporating or boiling, energy is required to overcome the remaining forces of attraction between the particles.
Evaporation vs. Boiling:
Evaporation occurs when particles leave a liquid from its surface only, without the liquid reaching its boiling point.
Boiling occurs when bubbles of gas form throughout the liquid. These bubbles rise to the surface and escape into the surroundings, turning the liquid into a gas.
Energy and Forces of Attraction:
- The amount of energy needed to change the state of a substance from solid to liquid, or from liquid to gas, depends on the strength of the forces between the particles.
- Stronger forces of attraction between particles require more energy to overcome, leading to higher melting and boiling points.
Melting and Boiling Points are Unique to Each Substance:
- Every substance has its own characteristic melting point and boiling point.
- The strength of the forces between particles determines these points:
- Ionic bonds in ionic solids are generally stronger than the forces between molecules in substances like water or hydrogen, leading to higher melting and boiling points. | Substance | Bonding Type | Melting Point | Boiling Point | |---|---|---|---| | Sodium chloride | Ionic | 801°C | 1413°C | | Water | Small molecules | 0°C | 100°C | | Hydrogen | Small molecules | -259°C | -252°C |
Evaporation Below Boiling Point: Evaporation can occur at temperatures below the boiling point of a substance.
Condensing and Freezing
Energy Release:
- When a substance condenses (gas to liquid) or freezes (liquid to solid), energy is transferred from the substance to the surroundings.
- This energy transfer occurs because the forces of attraction between particles become stronger as they move closer together in the solid or liquid state.
Predicting Physical State
The physical state of a substance at a given temperature can be predicted if its melting point and boiling point are known.
| Temperature | Predicted State |
|---|---|
| Given temperature < melting point | Solid |
| Given temperature between melting and boiling points | Liquid |
| Given temperature > boiling point | Gas |
500K+ Students Use These Powerful Tools to Master States of Matter For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
100 flashcards
Flashcards on States of Matter
Revise key concepts with interactive flashcards.
Try Chemistry Combined Science Flashcards10 quizzes
Quizzes on States of Matter
Test your knowledge with fun and engaging quizzes.
Try Chemistry Combined Science Quizzes29 questions
Exam questions on States of Matter
Boost your confidence with real exam questions.
Try Chemistry Combined Science Questions27 exams created
Exam Builder on States of Matter
Create custom exams across topics for better practice!
Try Chemistry Combined Science exam builder25 papers
Past Papers on States of Matter
Practice past papers to reinforce exam experience.
Try Chemistry Combined Science Past PapersOther Revision Notes related to States of Matter you should explore
Discover More Revision Notes Related to States of Matter to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Bonding & Substance Properties
Particle Theory & its Limitations
264+ studying
184KViews96%
114 rated
Bonding & Substance Properties
Properties of Ionic Compounds
341+ studying
190KViews96%
114 rated
Bonding & Substance Properties
Properties of Small Molecules
225+ studying
183KViews