Photo AI
Last Updated Sep 26, 2025
Reactions of Acids with Metals Simplified Revision Notes for GCSE AQA Chemistry Combined Science
Revision notes with simplified explanations to understand Reactions of Acids with Metals quickly and effectively.
403+ students studying
4.2.1 Reactions of Acids with Metals
When metals react with acids, a chemical reaction occurs that produces a salt and hydrogen gas. The reactivity of the metal determines how quickly and vigorously this reaction occurs.
General Reaction of Metals with Acids
The general word equation for the reaction between a metal and an acid is:
For example:
- Magnesium (Mg) reacts with hydrochloric acid (HCl) to produce magnesium chloride (MgCl₂) and hydrogen gas (H₂):
- Zinc (Zn) reacts with sulfuric acid (H₂SO₄) to produce zinc sulfate (ZnSO₄) and hydrogen gas (H₂):
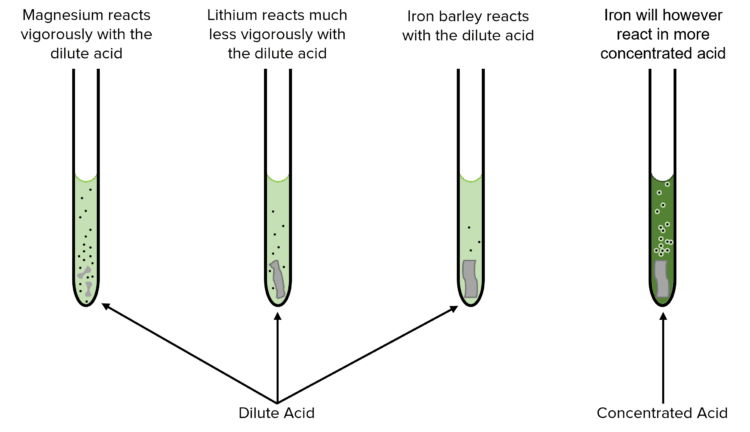
Reactivity and Reaction Speed
- Highly reactive metals, such as sodium or magnesium, react quickly and often violently with acids, releasing large amounts of hydrogen gas and heat.
- Less reactive metals, such as copper or silver, react much more slowly, if at all, with dilute acids.
The Reactivity Series helps predict these reactions. Metals that are higher in the series will react more readily with acids, while those lower in the series will react less readily.
Practical Applications
Understanding the reactions of metals with acids is important in various practical applications, such as:
- Industrial production of hydrogen gas.
- Corrosion prevention, where knowledge of reactivity helps in selecting materials that resist acidic environments.
- Metal extraction processes, where acids are used to dissolve metals from their ores.
500K+ Students Use These Powerful Tools to Master Reactions of Acids with Metals For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
80 flashcards
Flashcards on Reactions of Acids with Metals
Revise key concepts with interactive flashcards.
Try Chemistry Combined Science Flashcards8 quizzes
Quizzes on Reactions of Acids with Metals
Test your knowledge with fun and engaging quizzes.
Try Chemistry Combined Science Quizzes29 questions
Exam questions on Reactions of Acids with Metals
Boost your confidence with real exam questions.
Try Chemistry Combined Science Questions27 exams created
Exam Builder on Reactions of Acids with Metals
Create custom exams across topics for better practice!
Try Chemistry Combined Science exam builder25 papers
Past Papers on Reactions of Acids with Metals
Practice past papers to reinforce exam experience.
Try Chemistry Combined Science Past PapersOther Revision Notes related to Reactions of Acids with Metals you should explore
Discover More Revision Notes Related to Reactions of Acids with Metals to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Reactions of Acids
Metal & Acid Reactions as Redox Reactions
443+ studying
187KViews96%
114 rated
Reactions of Acids
Neutralisation of Acids and Salt Production
291+ studying
196KViews96%
114 rated
Reactions of Acids
Required Practical: Preparation of a Soluble Salt
462+ studying
194KViews