Photo AI
Last Updated Sep 26, 2025
The Process of Electrolysis Simplified Revision Notes for GCSE AQA Chemistry Combined Science
Revision notes with simplified explanations to understand The Process of Electrolysis quickly and effectively.
235+ students studying
4.3.1 The Process of Electrolysis
Electrolysis is a chemical process that uses an electric current to separate an ionic compound into its constituent ions, either when the compound is dissolved in solution or in a molten state. This process relies on electrostatic attractions, where ions in the substance are pulled in opposite directions by the electrical charge. The term "electrolysis" means "splitting using electricity."
Requirements for Electrolysis
For electrolysis to occur, the ionic compound must be either molten or dissolved in a solution. This is because the ions in a solid state are fixed in place within the crystal lattice and cannot move freely. In a liquid or dissolved state, the ions are free to move, allowing the process of electrolysis to take place.
The Setup for Electrolysis
.
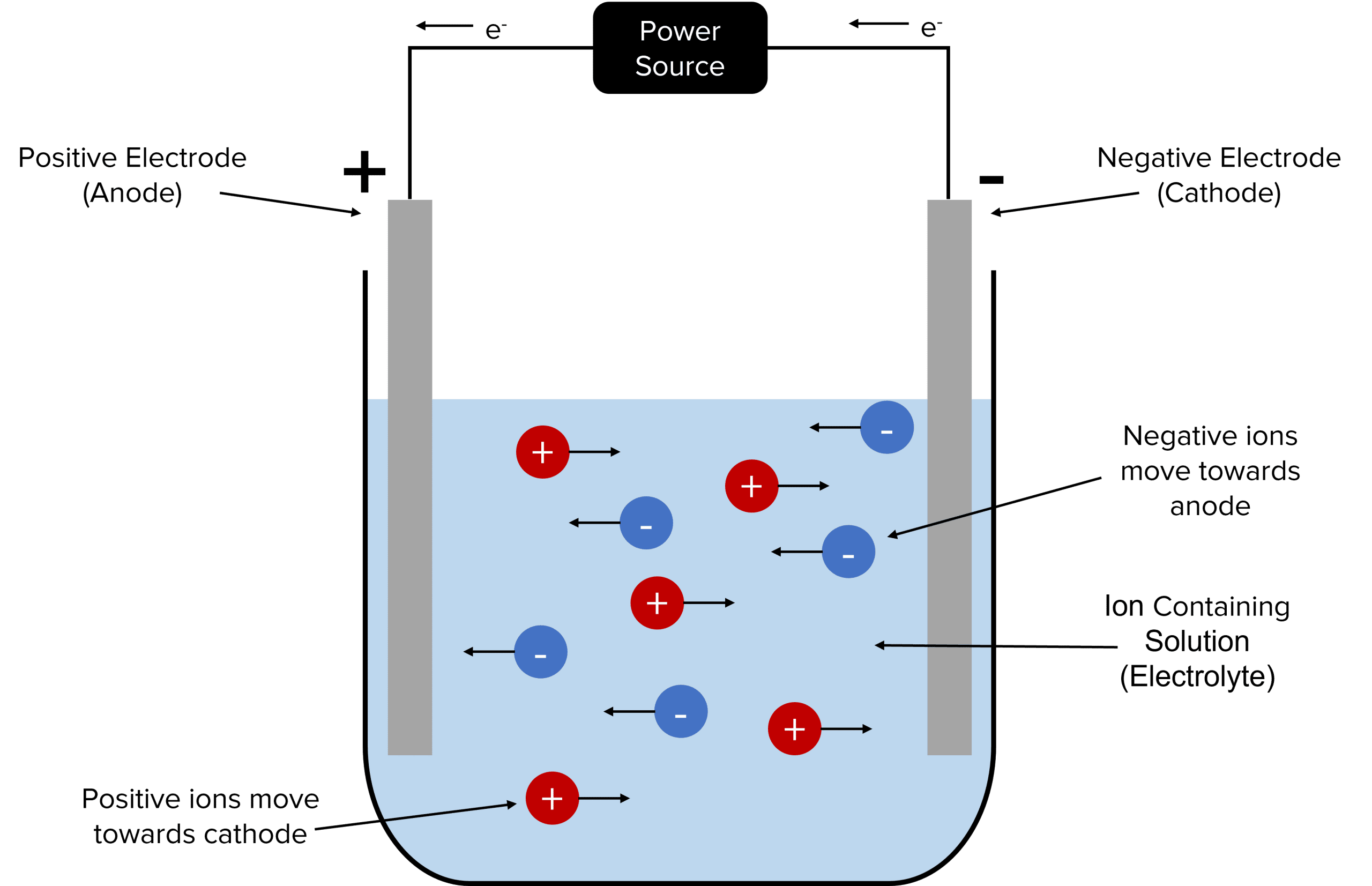
Key Components: To carry out electrolysis, the following components are needed:
- Electrolyte: The ionic compound in molten or solution form, which contains free-moving ions.
- Electrodes: Two electrodes are immersed in the electrolyte. The anode is the positive electrode, and the cathode is the negative electrode.
- Power Source: An electrical power source is connected to the electrodes, setting up a circuit and driving the flow of electric current. In most electrolysis setups, the electrodes are made of inert materials, meaning they do not react with the electrolyte. This ensures that the reactions occur only at the surface of the electrodes, without interference from the electrode materials.
How Electrolysis Works When the power source is turned on, it creates a potential difference between the electrodes, causing the ions in the electrolyte to move:
- At the Cathode (Negative Electrode):
- Positive ions in the electrolyte are attracted to the cathode. These ions move towards the cathode, where they gain electrons (reduction) from the external circuit.
- The reaction at the cathode typically looks like this:
- The positive ion is reduced and becomes a neutral atom, which is then discharged from the solution. This may result in the formation of a solid deposit or a gas, depending on the substance.
- At the Anode (Positive Electrode):
- Negative ions in the electrolyte are attracted to the anode. These ions move towards the anode, where they lose electrons (oxidation).
- The reaction at the anode typically looks like this:
- The negative ion is oxidised and becomes a neutral atom or molecule, which is discharged from the solution. This may also result in the release of a gas or the formation of a solid. The electrons released at the anode flow back to the cathode through the external circuit, maintaining the electrical flow.
Products of Electrolysis
Once ions are oxidised or reduced at the electrodes, they are discharged from the solution. The discharged species can leave the solution as:
- Solid sediments: Forming a layer on the electrode or falling to the bottom of the container.
- Gases: Escape from the electrolyte as bubbles that rise to the surface.
500K+ Students Use These Powerful Tools to Master The Process of Electrolysis For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
70 flashcards
Flashcards on The Process of Electrolysis
Revise key concepts with interactive flashcards.
Try Chemistry Combined Science Flashcards7 quizzes
Quizzes on The Process of Electrolysis
Test your knowledge with fun and engaging quizzes.
Try Chemistry Combined Science Quizzes29 questions
Exam questions on The Process of Electrolysis
Boost your confidence with real exam questions.
Try Chemistry Combined Science Questions27 exams created
Exam Builder on The Process of Electrolysis
Create custom exams across topics for better practice!
Try Chemistry Combined Science exam builder25 papers
Past Papers on The Process of Electrolysis
Practice past papers to reinforce exam experience.
Try Chemistry Combined Science Past PapersOther Revision Notes related to The Process of Electrolysis you should explore
Discover More Revision Notes Related to The Process of Electrolysis to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Electrolysis
Required Practical: Electrolysis of Aqueous Solutions
330+ studying
196KViews