Photo AI
Last Updated Sep 26, 2025
Reaction Profiles Simplified Revision Notes for GCSE AQA Chemistry Combined Science
Revision notes with simplified explanations to understand Reaction Profiles quickly and effectively.
409+ students studying
5.1.3 Reaction Profiles
Reaction profiles (also known as energy diagrams) are graphs that show how the energy of a system changes during a chemical reaction. These profiles help us understand whether a reaction is endothermic or exothermic and indicate the amount of energy required for the reaction to occur, known as the activation energy.
Understanding Reaction Profiles
- Y-Axis (Energy Level): Represents the energy of the system.
- X-Axis (Reaction Progress): Represents the progress of the reaction from reactants to products.
The key features of a reaction profile include:
- The energy levels of the reactants and products.
- The activation energy, which is the energy "hump" that must be overcome for the reaction to proceed.
- The overall energy change, which shows whether the reaction releases or absorbs energy.
Exothermic Reaction Profiles
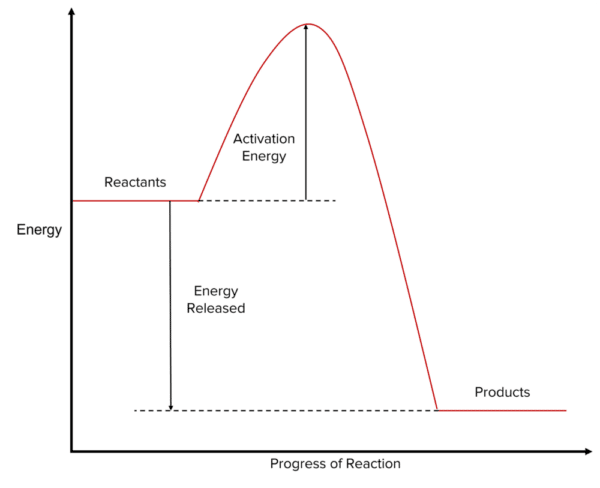
In exothermic reactions, the energy stored in the reactants is higher than the energy stored in the products. This means:
- The reactants are drawn at a higher level along the y-axis compared to the products.
- The difference in height between the reactants and products represents the energy released to the surroundings during the reaction.
The reaction profile includes a "hump," which represents the activation energy. This is the minimum energy required to start the reaction:
- The height of the hump above the reactants indicates the energy needed for the reactants to collide with sufficient force to react.
- Once the reactants overcome this activation energy, the reaction proceeds, releasing energy.
In an exothermic reaction, the overall energy change is negative because energy is lost as heat to the surroundings.
Endothermic Reaction Profiles
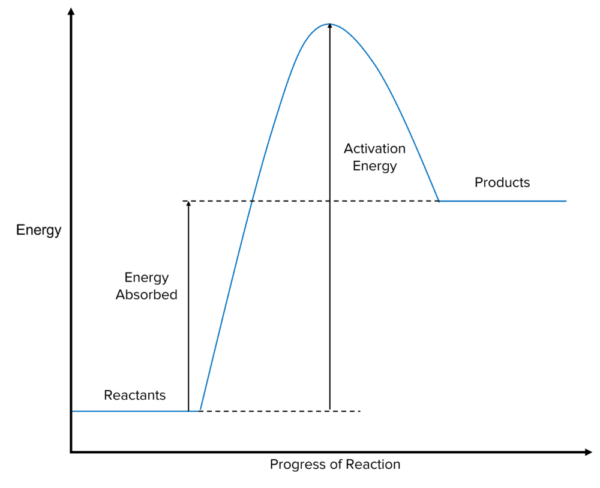
In endothermic reactions, the energy stored in the products is higher than the energy stored in the reactants. This means:
- The reactants are drawn at a lower level along the y-axis compared to the products.
- The difference in height between the reactants and products represents the energy absorbed from the surroundings during the reaction.
Like in exothermic reactions, the reaction profile features a hump representing the activation energy:
- The height of the hump above the reactants indicates the energy required for the reactants to collide and react.
- For the reaction to occur, the system must absorb enough energy to overcome this activation energy barrier.
In an endothermic reaction, the overall energy change is positive because the system absorbs energy from the surroundings.
500K+ Students Use These Powerful Tools to Master Reaction Profiles For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
50 flashcards
Flashcards on Reaction Profiles
Revise key concepts with interactive flashcards.
Try Chemistry Combined Science Flashcards5 quizzes
Quizzes on Reaction Profiles
Test your knowledge with fun and engaging quizzes.
Try Chemistry Combined Science Quizzes29 questions
Exam questions on Reaction Profiles
Boost your confidence with real exam questions.
Try Chemistry Combined Science Questions27 exams created
Exam Builder on Reaction Profiles
Create custom exams across topics for better practice!
Try Chemistry Combined Science exam builder25 papers
Past Papers on Reaction Profiles
Practice past papers to reinforce exam experience.
Try Chemistry Combined Science Past PapersOther Revision Notes related to Reaction Profiles you should explore
Discover More Revision Notes Related to Reaction Profiles to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Exothermic & Endothermic Reactions
Energy Transfer in Reactions
300+ studying
195KViews96%
114 rated
Exothermic & Endothermic Reactions
Required Practical: Investigating Temperature Changes
302+ studying
194KViews96%
114 rated
Exothermic & Endothermic Reactions
The Energy Change of Reactions
395+ studying
196KViews