Photo AI
Last Updated Sep 26, 2025
Group 1: The Alkali Metals Simplified Revision Notes for GCSE AQA Chemistry
Revision notes with simplified explanations to understand Group 1: The Alkali Metals quickly and effectively.
270+ students studying
1.2.5 Group 1: The Alkali Metals
Physical and Chemical Properties
Physical Properties:
- All metals look dull on the outside due to the formation of a layer of oxide.
- Over time, this layer of oxide makes the metal look dull.
- The inside of every metal is shiny.
- It is possible to cut every metal with a knife.
- Alkali metals are kept in oil to prevent them from reacting with oxygen and moisture in the air.
- They have low density, so most alkali metals float.
- Their boiling point and melting point are lower than many other metals.
Reactivity:
- All Group 1 metals have 1 electron in their outer shell, making them very reactive as they bond by losing this electron.
- Reactivity increases down the group because the outer electron is more easily lost, as it's further from the nucleus.
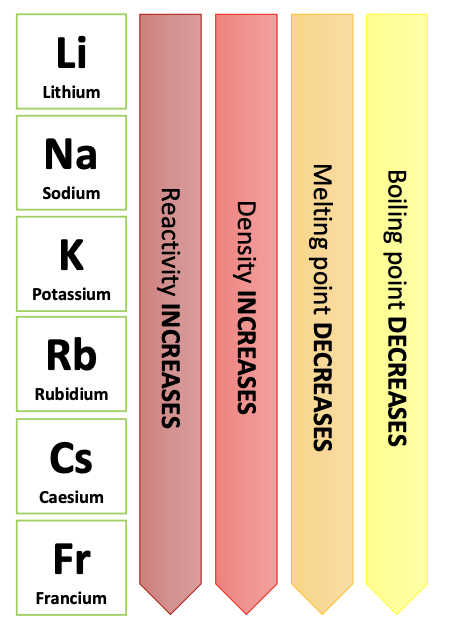
Trends in Group 1:
- Reactivity INCREASES down the group.
- Density INCREASES down the group.
- Melting point DECREASES down the group.
- Boiling point DECREASES down the group.
Chemical Properties
Reaction with Oxygen to Form Oxides:
Example (Potassium):
The oxide layer that makes the metal look dull forms more quickly as you move down the group.
Reaction with Water to Form Hydroxides (Alkalis):
Example (Lithium):
- The metals create an alkali as they react with water (e.g., turning purple with a universal indicator).
- The reaction produces hydrogen gas.
- The metal floats, moves, and fizzes on the water's surface.
- Sodium: Moves quicker and forms a ball shape.
- Potassium: Moves quickly and has a lilac flame.
500K+ Students Use These Powerful Tools to Master Group 1: The Alkali Metals For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
70 flashcards
Flashcards on Group 1: The Alkali Metals
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards7 quizzes
Quizzes on Group 1: The Alkali Metals
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Group 1: The Alkali Metals
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Group 1: The Alkali Metals
Create custom exams across topics for better practice!
Try Chemistry exam builder26 papers
Past Papers on Group 1: The Alkali Metals
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Group 1: The Alkali Metals you should explore
Discover More Revision Notes Related to Group 1: The Alkali Metals to Deepen Your Understanding and Improve Your Mastery
