Photo AI
Last Updated Sep 26, 2025
Le Chatelier’s Principle: Pressure Changes on Equilibrium Simplified Revision Notes for GCSE AQA Chemistry
Revision notes with simplified explanations to understand Le Chatelier’s Principle: Pressure Changes on Equilibrium quickly and effectively.
494+ students studying
6.2.7 Le Chatelier's Principle: Pressure Changes on Equilibrium
The Effect of Pressure Changes on Equilibrium
According to Le Chatelier's Principle, an equilibrium will shift its position to oppose any change in pressure.
This means that, when the pressure is increased, the equilibrium will shift towards whichever side of the reaction has fewer moles of gas. This reduces the pressure of the reaction and opposes the change.
-
In reactions involving gases, an increase in pressure will cause the equilibrium to shift towards the side with fewer moles of gas, reducing the pressure.
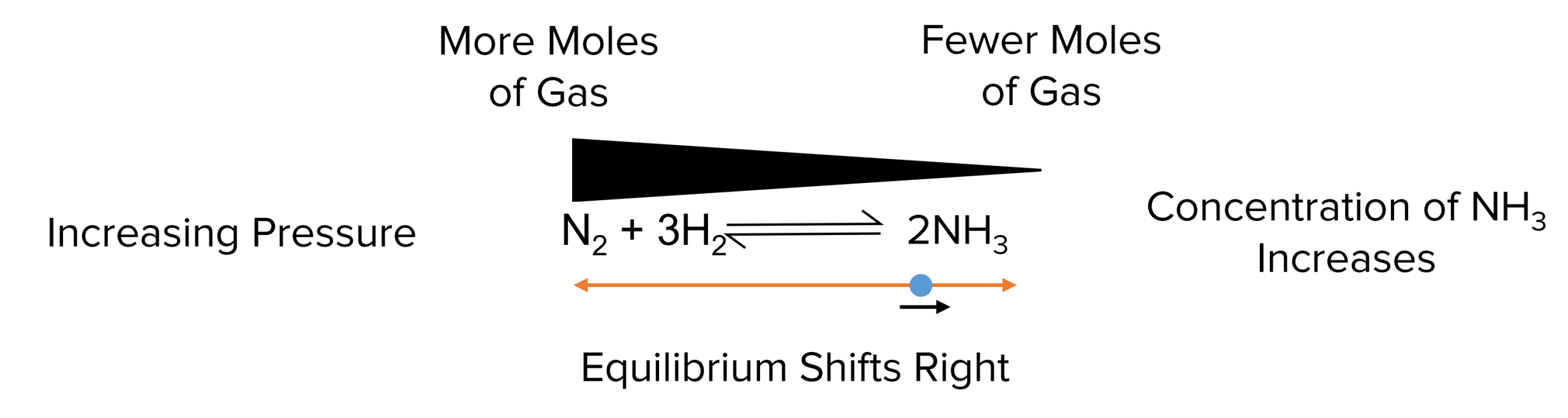
-
Whereas, a decrease in pressure will cause the equilibrium to shift towards the side with more moles of gas, increasing the pressure.
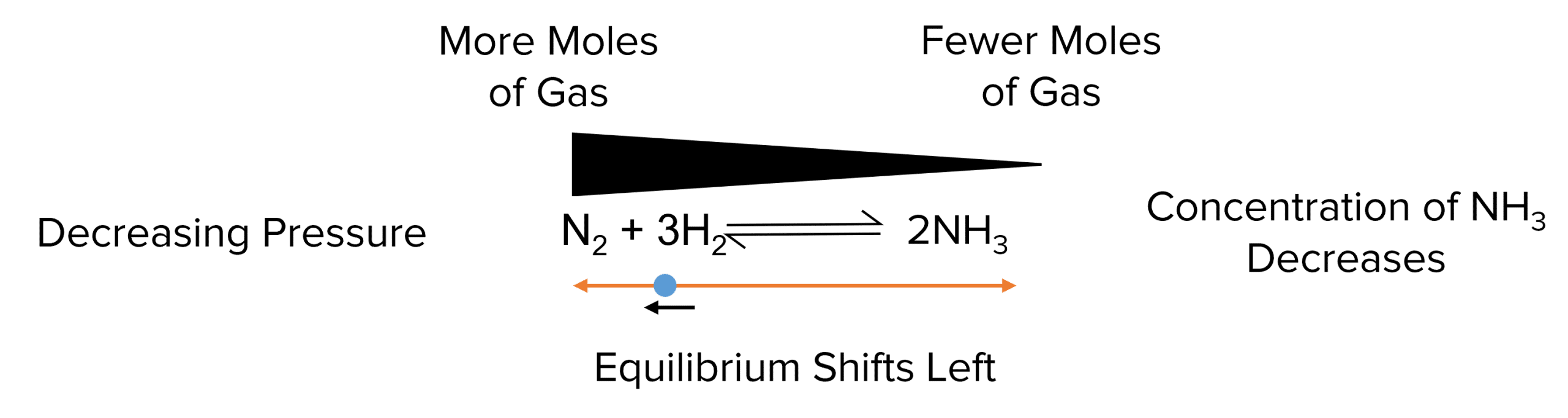
Example: For the ammonia formation reaction:
500K+ Students Use These Powerful Tools to Master Le Chatelier’s Principle: Pressure Changes on Equilibrium For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
80 flashcards
Flashcards on Le Chatelier’s Principle: Pressure Changes on Equilibrium
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards8 quizzes
Quizzes on Le Chatelier’s Principle: Pressure Changes on Equilibrium
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Le Chatelier’s Principle: Pressure Changes on Equilibrium
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Le Chatelier’s Principle: Pressure Changes on Equilibrium
Create custom exams across topics for better practice!
Try Chemistry exam builder26 papers
Past Papers on Le Chatelier’s Principle: Pressure Changes on Equilibrium
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Le Chatelier’s Principle: Pressure Changes on Equilibrium you should explore
Discover More Revision Notes Related to Le Chatelier’s Principle: Pressure Changes on Equilibrium to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Reversibility & Equilibrium
Energy Changes & Reversible Reactions
395+ studying
195KViews96%
114 rated
Reversibility & Equilibrium
The Effect of Changing Conditions on Equilibrium
420+ studying
198KViews