Photo AI
Last Updated Sep 26, 2025
Conservation of Energy Simplified Revision Notes for GCSE AQA Physics Combined Science
Revision notes with simplified explanations to understand Conservation of Energy quickly and effectively.
323+ students studying
1.1.11 Conservation of Energy
Conservation of Energy
Energy can be transferred, stored, or dissipated (wasted). However, energy cannot be created or destroyed.
This means that in a closed system (where energy cannot enter or leave), the net change in energy will always be zero.
Useful and Wasted Energy
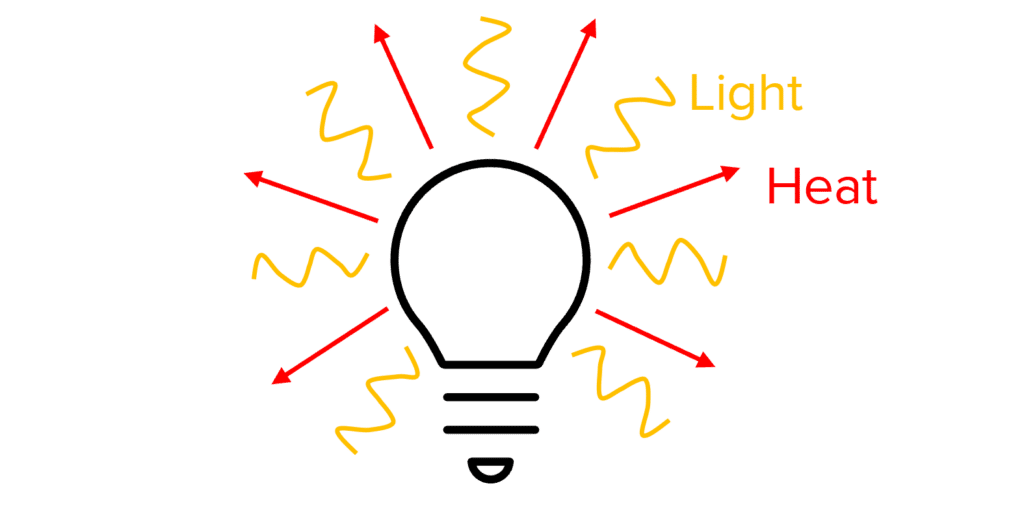
When energy is transferred between stores, not all of it will be directed to the intended energy store. The energy that is successfully transferred to the desired store is called useful energy. The energy that ends up in other stores is termed wasted energy. We say that wasted energy has been dissipated.
For example, a light bulb emits both heat and light. The energy used to produce light is useful energy, while the energy that goes into heating the bulb is wasted, as the goal is not to heat the bulb.
The useful and wasted energy always add up to the energy input because energy cannot be created or destroyed.
Minimising Energy Loss
There are several strategies to reduce unwanted energy transfers. Two key methods for minimising wasted energy are thermal insulation and lubrication.
Thermal Insulation
Energy often escapes from a system as heat, transferring to the surroundings. By surrounding an object with one or more layers of material, you can slow down the rate of heat loss. This process is known as insulation. For instance, when you feel cold, you might put on a coat. The coat helps retain the heat energy your body generates, preventing it from escaping into the air around you, which keeps you warm longer.
Lubrication
Energy can also be lost due to friction when objects rub against each other. The amount of energy lost to friction can be reduced by making the surfaces smoother, a process known as lubrication. For example, roads become much more slippery when icy because ice is smoother than tarmac, reducing the friction between car tyres and the road.

Thermal Conductivity
Some materials make better thermal insulators than others. This is because of a property called thermal conductivity. Thermal conductivity tells you how well a material transfers heat energy through it.
Materials with a low thermal conductivity transfer less heat energy and therefore make good thermal insulators. Materials with a high thermal conductivity transfer more heat energy and therefore make poor thermal insulators.
For example, if you heat one end of a metal rod, the other end will also get hot. This is because metal has a high thermal conductivity and so the heat is transferred quickly to the metal. However, if you heat one end of a plastic rod, the other end will stay cool because plastic has low thermal conductivity.
Buildings are constructed with materials with a low thermal conductivity in order to insulate the rooms inside. This allows the building to stay warm in cold weather but cool in the summer.
500K+ Students Use These Powerful Tools to Master Conservation of Energy For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
150 flashcards
Flashcards on Conservation of Energy
Revise key concepts with interactive flashcards.
Try Physics Combined Science Flashcards15 quizzes
Quizzes on Conservation of Energy
Test your knowledge with fun and engaging quizzes.
Try Physics Combined Science Quizzes29 questions
Exam questions on Conservation of Energy
Boost your confidence with real exam questions.
Try Physics Combined Science Questions27 exams created
Exam Builder on Conservation of Energy
Create custom exams across topics for better practice!
Try Physics Combined Science exam builder24 papers
Past Papers on Conservation of Energy
Practice past papers to reinforce exam experience.
Try Physics Combined Science Past PapersOther Revision Notes related to Conservation of Energy you should explore
Discover More Revision Notes Related to Conservation of Energy to Deepen Your Understanding and Improve Your Mastery
