Photo AI
Last Updated Sep 26, 2025
Heating & Cooling Graphs Simplified Revision Notes for GCSE AQA Physics Combined Science
Revision notes with simplified explanations to understand Heating & Cooling Graphs quickly and effectively.
238+ students studying
3.2.5 Heating & Cooling Graphs
For your exams, you must be able to interpret heating and cooling graphs.
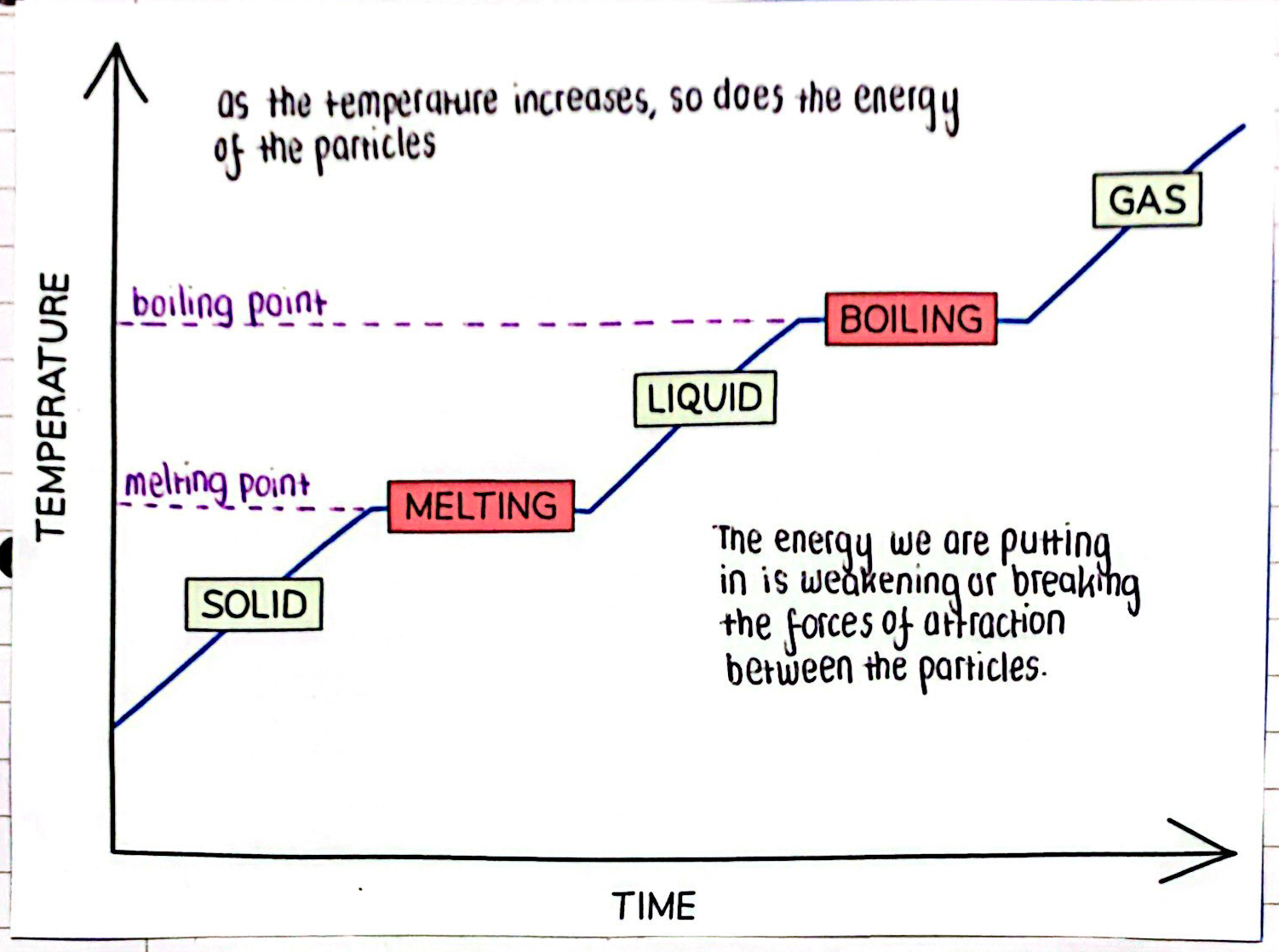
Heating Graph We are going to discuss the heating graph step by step:
- Solid Heating – as temperature and time increases, the solid is starting to heat up.
- Melting – once the solid reaches its melting point (in this case it is 0 degrees), it starts to melt and turn into a liquid.
- Liquid Heating – as temperature and time increases, the liquid is starting to heat up.
- Boiling – once the liquid reaches its boiling point (in this case, it is 100 degrees), it starts to evaporate and turn into a gas.
The straight lines on the graph are very important. These are points where the substance is being heated, but instead of increasing in temperature, the substance is changing state.
:::
Cooling Graph Now, we are going to discuss the cooling graph step by step:
- Gas cooling – the gas is being cooled down as time goes on.
- Gas to liquid – once the gas reaches its condensation point, the gas condenses into a liquid.
- Liquid cooling – the liquid is being cooled down as time goes on.
- Liquid to solid – once the liquid reaches its freezing point, the liquid freezes into a solid.
The straight lines on the graph are very important. These are points where the substance is being cooled, but instead of decreasing in temperature, the substance is changing state.
:::
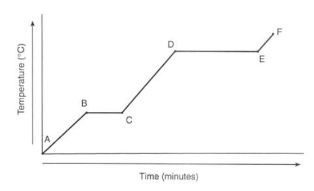
The graph shows the temperature of ice:
- At A it is Solid.
- At B, it reaches 0°C.
- From B to C there is no temperature change because the energy is used through melting.
- From C to D it is in liquid state.
- From D to E the water is boiling. This takes longer, because evaporation takes more energy
- From E to F the gas is heating.
Important info:
- Energy is absorbed when melting and evaporating and energy is released when freezing and condensing.
- Sublimation is when a solid goes straight to gas – "dry ice" (solid CO2 does this)
500K+ Students Use These Powerful Tools to Master Heating & Cooling Graphs For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
70 flashcards
Flashcards on Heating & Cooling Graphs
Revise key concepts with interactive flashcards.
Try Physics Combined Science Flashcards7 quizzes
Quizzes on Heating & Cooling Graphs
Test your knowledge with fun and engaging quizzes.
Try Physics Combined Science Quizzes29 questions
Exam questions on Heating & Cooling Graphs
Boost your confidence with real exam questions.
Try Physics Combined Science Questions27 exams created
Exam Builder on Heating & Cooling Graphs
Create custom exams across topics for better practice!
Try Physics Combined Science exam builder24 papers
Past Papers on Heating & Cooling Graphs
Practice past papers to reinforce exam experience.
Try Physics Combined Science Past PapersOther Revision Notes related to Heating & Cooling Graphs you should explore
Discover More Revision Notes Related to Heating & Cooling Graphs to Deepen Your Understanding and Improve Your Mastery
