Photo AI
Last Updated Sep 26, 2025
Positive Ions Simplified Revision Notes for GCSE AQA Physics Combined Science
Revision notes with simplified explanations to understand Positive Ions quickly and effectively.
467+ students studying
4.1.5 Positive Ions
Atoms are generally neutral, with the same number of protons in the nucleus as electrons orbiting around it.
However, atoms can lose or gain electrons due to various interactions, forming charged particles known as ions:
- If an atom loses one or more electrons, it becomes a positively charged ion.
- If an atom gains one or more electrons, it becomes a negatively charged ion.
For example, a helium atom has two electrons orbiting its nucleus.
The atom is neutral as it has two positive protons and two negative electrons.
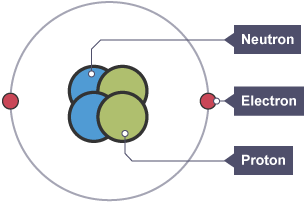
A helium atom that has lost or gained an electron is an ion.
If the ion has two positive protons but one negative electron, it is a positive ion.
If the ion has two positive protons but three negative electrons, it is a negative ion.
500K+ Students Use These Powerful Tools to Master Positive Ions For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
110 flashcards
Flashcards on Positive Ions
Revise key concepts with interactive flashcards.
Try Physics Combined Science Flashcards11 quizzes
Quizzes on Positive Ions
Test your knowledge with fun and engaging quizzes.
Try Physics Combined Science Quizzes29 questions
Exam questions on Positive Ions
Boost your confidence with real exam questions.
Try Physics Combined Science Questions27 exams created
Exam Builder on Positive Ions
Create custom exams across topics for better practice!
Try Physics Combined Science exam builder24 papers
Past Papers on Positive Ions
Practice past papers to reinforce exam experience.
Try Physics Combined Science Past PapersOther Revision Notes related to Positive Ions you should explore
Discover More Revision Notes Related to Positive Ions to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Atoms & Isotopes
The Absorption & Emission of EM Radiation
497+ studying
194KViews