Photo AI
Last Updated Sep 26, 2025
Changes in the Atomic Model Simplified Revision Notes for GCSE AQA Physics Combined Science
Revision notes with simplified explanations to understand Changes in the Atomic Model quickly and effectively.
398+ students studying
4.1.10 Changes in the Atomic Model
Alpha-Scattering Experiment
Historical Context
- Ancient Greeks:
- Believed everything is made of atoms.
- Atoms are tiny spheres that cannot be divided.
- 1897: Discovery of Electrons
- Scientists discovered that atoms contain tiny negative particles called electrons.
- This showed that atoms are not spheres that cannot be divided and have an internal structure.
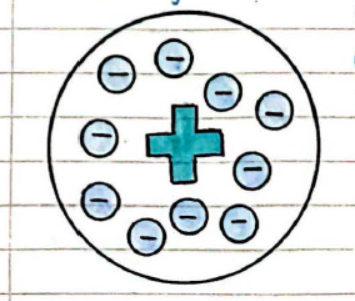
Plum-Pudding Model
- Suggested that an atom is a ball of positive charge with negative electrons embedded in it.
- The alpha-scattering experiment was carried out to test if this model was correct.
Alpha-Scattering Experiment
- Setup:
- Scientists took a piece of gold foil, which can be hammered into a very thin foil, just a few atoms thick.
- They then fired tiny alpha particles at the gold foil. Alpha particles have a positive charge.
- Observations:
- Most alpha particles passed straight through the gold foil without changing direction.
- Some alpha particles were deflected (changed direction as they passed through).
- Sometimes an alpha particle bounced straight back off the gold foil.
Conclusion:
- Most alpha particles went straight through the gold atoms:
- Therefore, atoms are mainly empty space (the plum-pudding model was wrong).
- Some alpha particles were deflected:
- Therefore, the centre of an atom must have a positive charge. Alpha particles that come close to this area are repelled and change direction.
- Sometimes an alpha particle bounced straight back:
- The centre of an atom must contain a great deal of mass. This central part of an atom is now called the nucleus.
- Impact:
- Scientists replaced the plum-pudding model with the nuclear model.
The Nuclear Model
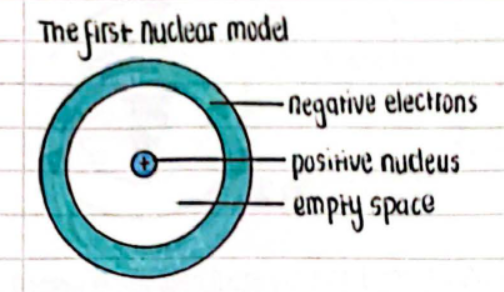
The First Nuclear Model
- Components:
- Negative electrons
- Positive nucleus
- Empty space
- Description:
- Most of the atom is simply empty space.
Niels Bohr's Model
- Electrons:
- Electrons orbit the nucleus at specific distances.
- These orbits are now called energy levels or shells.
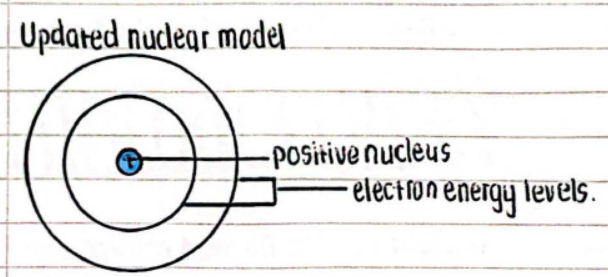
Updated Nuclear Model
- Components:
- Positive nucleus
- Electron energy levels (shells)
Protons and Neutrons
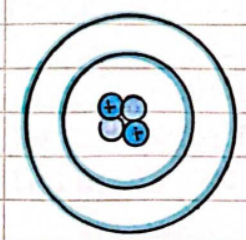
- Protons:
- The positive charge in the nucleus is due to tiny positive particles called protons.
- Neutrons:
- Discovered by James Chadwick; the nucleus also contains neutral particles called neutrons.
Atomic Structure
- Atoms have no overall charge because the number of electrons is the same as the number of protons.
- The radius of an atom:
- The radius of the nucleus:
Subatomic Particles
| Particle | Relative Charge | Relative Mass |
|---|---|---|
| Proton | +1 | 1 |
| Neutron | 0 | 1 |
| Electron | -1 | Very small |
500K+ Students Use These Powerful Tools to Master Changes in the Atomic Model For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
110 flashcards
Flashcards on Changes in the Atomic Model
Revise key concepts with interactive flashcards.
Try Physics Combined Science Flashcards11 quizzes
Quizzes on Changes in the Atomic Model
Test your knowledge with fun and engaging quizzes.
Try Physics Combined Science Quizzes29 questions
Exam questions on Changes in the Atomic Model
Boost your confidence with real exam questions.
Try Physics Combined Science Questions27 exams created
Exam Builder on Changes in the Atomic Model
Create custom exams across topics for better practice!
Try Physics Combined Science exam builder24 papers
Past Papers on Changes in the Atomic Model
Practice past papers to reinforce exam experience.
Try Physics Combined Science Past PapersOther Revision Notes related to Changes in the Atomic Model you should explore
Discover More Revision Notes Related to Changes in the Atomic Model to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Atoms & Isotopes
The Absorption & Emission of EM Radiation
477+ studying
197KViews