Photo AI
Last Updated Sep 26, 2025
Changes in Energy Simplified Revision Notes for GCSE AQA Physics
Revision notes with simplified explanations to understand Changes in Energy quickly and effectively.
368+ students studying
1.1.8 Changes in Energy
Energy Transfer
When an object is heated, thermal energy is transferred to it, resulting in an increase in the object's temperature. For example, when you heat a pan of soup over a flame, the thermal energy from the flame is absorbed by the soup, causing its temperature to rise.
The amount of energy required to heat different objects by a certain amount can be calculated using the principles of specific heat capacity.
Specific Heat Capacity
Specific heat capacity refers to the amount of energy required to raise the temperature of 1 kilogram of material by 1°C. This property is denoted by the lowercase letter c and is measured in J/kg°C.
Study Tip
- Materials with a high specific heat capacity require more thermal energy to increase their temperature.
- Materials with a low specific heat capacity need less thermal energy to achieve the same temperature increase.
The formula for Heat Change To calculate the amount of thermal energy needed to increase the temperature of an object, we use the formula:
where:
- is the change in thermal energy (Joules, J),
- is the mass of the object (kilograms, kg),
- is the specific heat capacity of the material (J/kg°C),
- is the change in temperature (degrees Celsius, °C or Kelvin, K).
Worked Example If you want to calculate the energy required to heat 2 kg of water (with a specific heat capacity of 4,200 J/kg°C) from 20°C to 30°C:
This means 84,000 joules of energy are needed to raise the temperature of the water by 10°C.
Required Practical Investigation into Specific Heat Capacity
Conducting the Experiment
- Measure the mass of the block using a balance.
- Place the block in a container surrounded by insulating material (e.g., bubble wrap) to minimize heat loss.
- Insert a thermometer into the container, and connect a power supply, heater, and ammeter to the block as shown in the diagram.
- Record the starting temperature of the block.
- Turn on the power supply and start a stopwatch simultaneously.
- Record the current (I) from the ammeter and the potential difference (V) from the power source.
- After 10 minutes, measure the final temperature of the block.
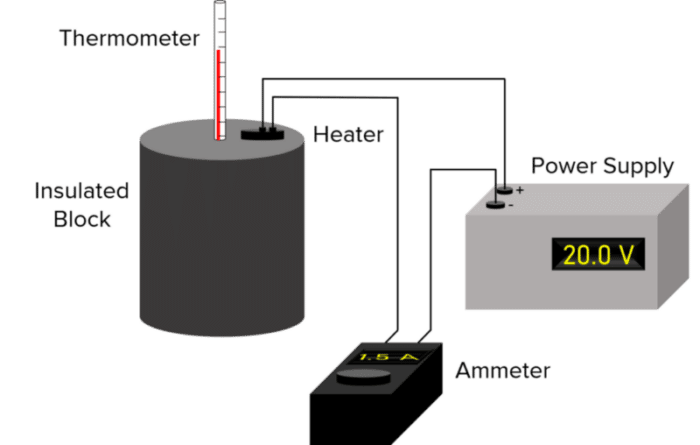
Calculating the Specific Heat Capacity 8. Calculate the change in temperature using:
- Find the power of the heater using the equation:
- Calculate the energy transferred to the block:
where t is the time in seconds (600 s for 10 minutes).
-
6 3Assuming no heat is lost to the surroundings (due to insulation), this energy is the amount transferred to the block, so
-
Rearrange the specific heat capacity formula to solve for
- Substitute the values for and into the equation to find the specific heat capacity.
Real-World Applications
- Water has a high specific heat capacity, making it effective for use in cooling systems and radiators because it can absorb a lot of heat without a large temperature increase.
- Metal utensils heat up quickly due to their low specific heat capacity, which makes them efficient for cooking but also means they need to be handled carefully to avoid burns.
500K+ Students Use These Powerful Tools to Master Changes in Energy For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
160 flashcards
Flashcards on Changes in Energy
Revise key concepts with interactive flashcards.
Try Physics Flashcards16 quizzes
Quizzes on Changes in Energy
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes15 questions
Exam questions on Changes in Energy
Boost your confidence with real exam questions.
Try Physics Questions223 exams created
Exam Builder on Changes in Energy
Create custom exams across topics for better practice!
Try Physics exam builder25 papers
Past Papers on Changes in Energy
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Changes in Energy you should explore
Discover More Revision Notes Related to Changes in Energy to Deepen Your Understanding and Improve Your Mastery
