Photo AI
Last Updated Sep 26, 2025
Specific Heat Capacity Simplified Revision Notes for GCSE AQA Physics
Revision notes with simplified explanations to understand Specific Heat Capacity quickly and effectively.
285+ students studying
3.2.2 Specific Heat Capacity
Specific Heat Capacity
Specific heat capacity (c) refers to how well a substance stores heat.
It is the amount of energy needed to raise the temperature of 1 kg of a substance by 1°C.
This depends on the material of the substance, its mass, and the energy added. The formula for calculating the change in thermal energy is: change in thermal energy = mass × specific heat capacity × temperature change
Where ∆E is the change in thermal energy, in joules J, specific heat capacity, c in joules per kilogram per degree Celcius , mass m in kilograms kg and temperature change ∆T in degrees Celcius °C.
Rearranged Formula:
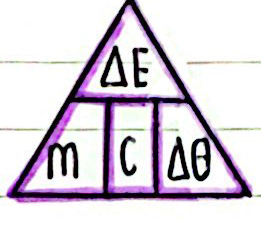
Table of Variables
| Variable | Symbol | Units |
|---|---|---|
| Change in Energy | Joules (J) | |
| Mass | Kilograms (kg) | |
| Specific Heat Capacity | J/kg°C | |
| Change in Temperature | Degrees Celsius (°C) |
500K+ Students Use These Powerful Tools to Master Specific Heat Capacity For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
60 flashcards
Flashcards on Specific Heat Capacity
Revise key concepts with interactive flashcards.
Try Physics Flashcards6 quizzes
Quizzes on Specific Heat Capacity
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes16 questions
Exam questions on Specific Heat Capacity
Boost your confidence with real exam questions.
Try Physics Questions4 exams created
Exam Builder on Specific Heat Capacity
Create custom exams across topics for better practice!
Try Physics exam builder25 papers
Past Papers on Specific Heat Capacity
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Specific Heat Capacity you should explore
Discover More Revision Notes Related to Specific Heat Capacity to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Internal Energy & Energy Transfers
Heating & Cooling Graphs
285+ studying
200KViews