Photo AI
Last Updated Sep 27, 2025
Calculating Rates of Reactions Simplified Revision Notes for GCSE Edexcel Chemistry Combined Science
Revision notes with simplified explanations to understand Calculating Rates of Reactions quickly and effectively.
458+ students studying
6.1.1 Calculating Rates of Reactions
What is the Rate of Reaction?
The rate of a chemical reaction tells us how quickly the reaction happens. It measures how fast the reactants are used up or how quickly the products are formed during a reaction.
Rates of reaction are important because they help us understand how efficient a reaction is, which is particularly useful in industrial processes
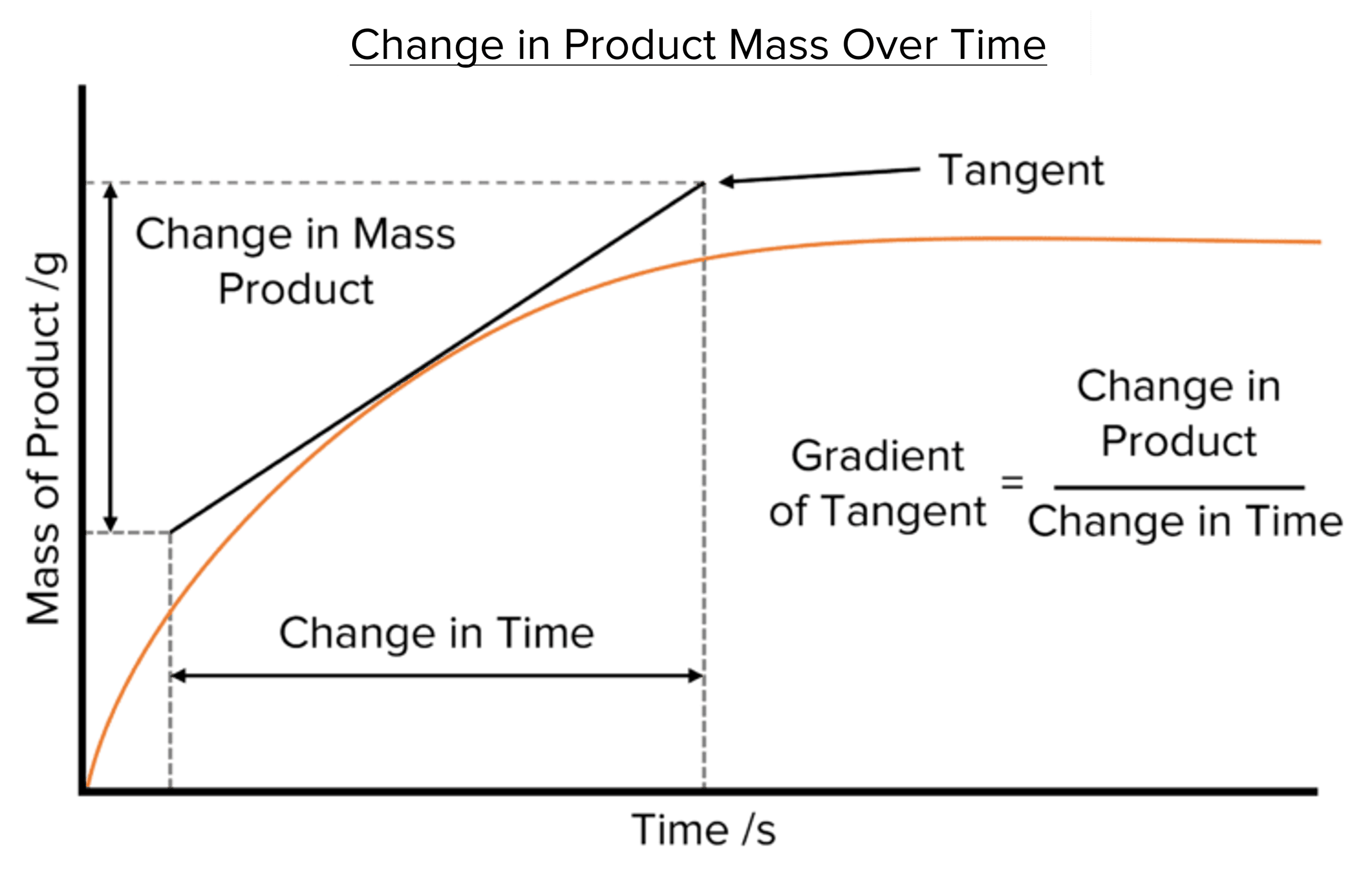
Calculating Mean Rate of Reaction The mean rate of reaction can be calculated using one of two formulas:
-
Using Reactants
-
Using Products:
A higher rate indicates a faster reaction, while a lower rate suggests a slower reaction.
Units:
- The amount of reactant used or product formed is usually measured in grams (g) or cubic centimetres (cm³).
- Time is measured in seconds (s).
- Therefore, the rate is expressed in units like g/s or cm³/s.
Example Question: Calculating the Mean Rate of Reaction A chemical reaction between magnesium and hydrochloric acid produces hydrogen gas. During an experiment, 0.12 grams of magnesium were completely consumed in 45 seconds, producing 100 cm³ of hydrogen gas.
Tasks:
- Calculate the mean rate of reaction using the amount of reactant (magnesium) consumed.
- Calculate the mean rate of reaction using the amount of product (hydrogen gas) formed.
- Determine which calculation indicates a higher rate of reaction and explain why. Given Data:
-
Amount of magnesium used = 0.12 g
-
Amount of hydrogen gas formed = 100 cm³
-
Time taken = 45 s Units:
-
Rate using magnesium: g/s
-
Rate using hydrogen gas: cm³/s
Answers
- Rate using magnesium: 0.00267 g/s
- Rate using hydrogen gas: 2.22 cm³/s
- Higher rate of reaction: The rate using hydrogen gas (2.22 cm³/s) is higher because it reflects the volume of gas produced, which occurs more quickly and in greater quantity compared to the mass of magnesium consumed.
500K+ Students Use These Powerful Tools to Master Calculating Rates of Reactions For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
10 flashcards
Flashcards on Calculating Rates of Reactions
Revise key concepts with interactive flashcards.
Try Chemistry Combined Science Flashcards1 quizzes
Quizzes on Calculating Rates of Reactions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Combined Science Quizzes13 questions
Exam questions on Calculating Rates of Reactions
Boost your confidence with real exam questions.
Try Chemistry Combined Science Questions27 exams created
Exam Builder on Calculating Rates of Reactions
Create custom exams across topics for better practice!
Try Chemistry Combined Science exam builder28 papers
Past Papers on Calculating Rates of Reactions
Practice past papers to reinforce exam experience.
Try Chemistry Combined Science Past PapersOther Revision Notes related to Calculating Rates of Reactions you should explore
Discover More Revision Notes Related to Calculating Rates of Reactions to Deepen Your Understanding and Improve Your Mastery
Load more notes