Photo AI
Last Updated Sep 27, 2025
Alkanes Simplified Revision Notes for GCSE Edexcel Chemistry
Revision notes with simplified explanations to understand Alkanes quickly and effectively.
226+ students studying
Alkanes
Hydrocarbons are compounds made of only carbon and hydrogen atoms. They can be alkanes or alkenes, depending on the type of bonds between the carbon atoms.
- Alkanes have single bonds between all the carbon atoms. This means they are saturated because they have bonded with as many atoms as possible.
Alkanes (Saturated Hydrocarbons)
Alkanes belong to a group called a homologous series. This just means they are a family of molecules that follow a pattern. Alkanes are saturated because their carbon atoms are bonded to as many hydrogen atoms as possible.
The general formula for alkanes is CnH₂n+₂, meaning for every carbon atom, there are two hydrogen atoms plus two more.
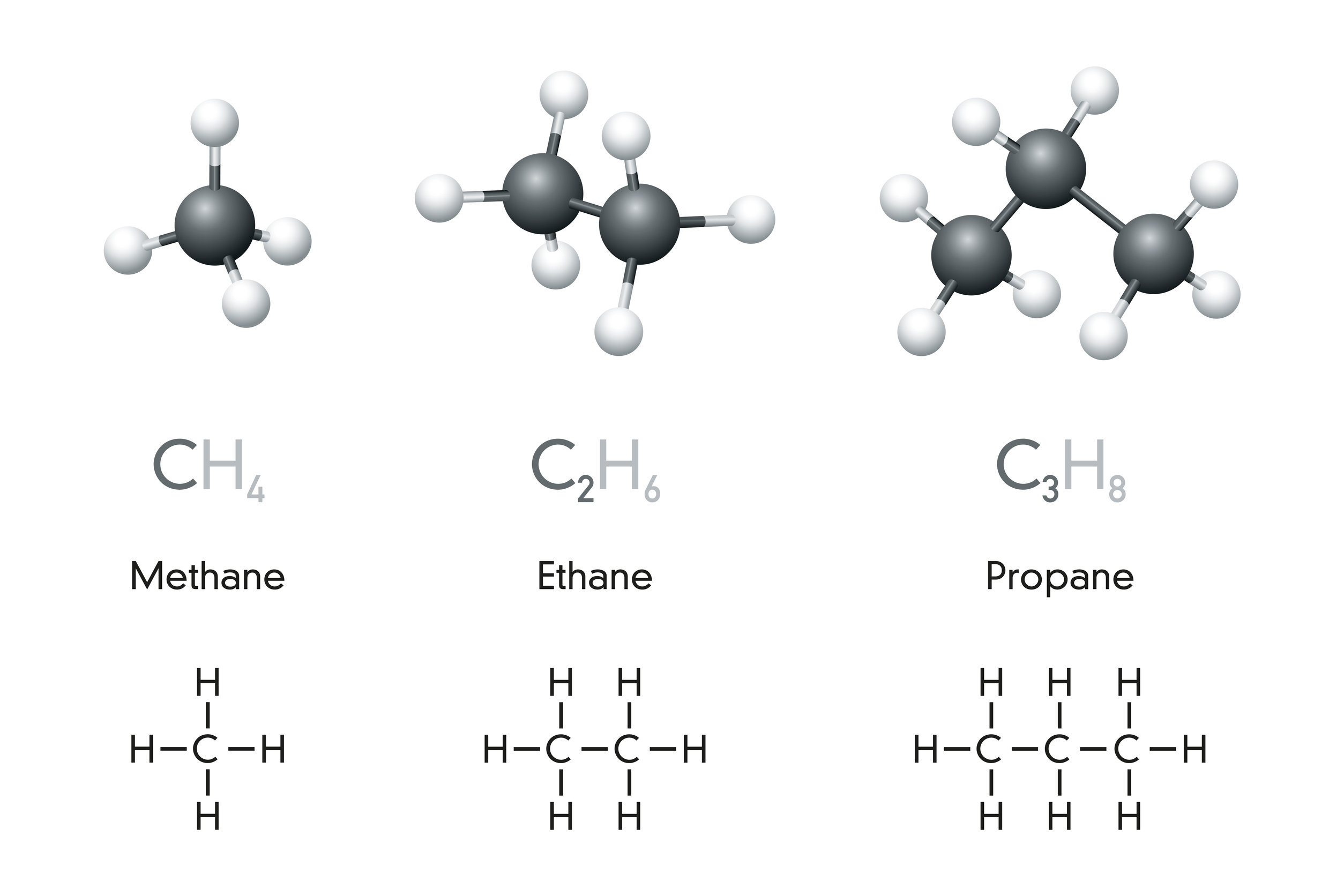
- The first four alkanes—methane, ethane, propane, and butane—are gases at room temperature.
Why Do the Boiling Points of Alkanes Increase with Chain Length?
Longer molecules have more surface area to stick to each other, so it takes more heat to separate them. This is why longer alkanes have higher boiling points.
Reactivity of Alkanes
The bonds between carbon and hydrogen in alkanes are very strong, so alkanes don't react easily with other substances.
However, alkanes will burn in oxygen, releasing a lot of heat energy.
Combustion Reactions
Alkanes and alkenes can burn in oxygen, which is called combustion. This is why they are used as fuels.
Complete combustion happens when there is plenty of oxygen, producing carbon dioxide (CO₂) and water (H₂O).
Incomplete combustion occurs when there isn't enough oxygen. This produces carbon monoxide (CO), which is dangerous because it can stop your blood from carrying oxygen. If oxygen is really low, soot (black carbon particles) may also be produced.
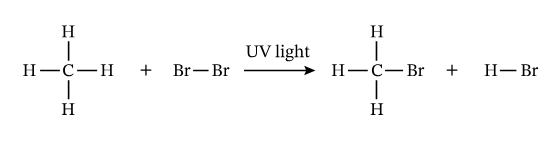
Reactions of Alkanes with Bromine
Alkanes only react with bromine if UV light is present. Since alkanes are already fully bonded to hydrogen, one hydrogen atom is replaced by a bromine atom in a substitution reaction.
500K+ Students Use These Powerful Tools to Master Alkanes For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
20 flashcards
Flashcards on Alkanes
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards16 questions
Exam questions on Alkanes
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Alkanes
Create custom exams across topics for better practice!
Try Chemistry exam builder78 papers
Past Papers on Alkanes
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Alkanes you should explore
Discover More Revision Notes Related to Alkanes to Deepen Your Understanding and Improve Your Mastery
Load more notes