Photo AI
Last Updated Sep 26, 2025
Specific heat capacity Simplified Revision Notes for GCSE Edexcel Physics Combined Science
Revision notes with simplified explanations to understand Specific heat capacity quickly and effectively.
321+ students studying
Specific heat capacity
Specific Heat Capacity
Specific heat capacity (c) refers to how well a substance stores heat.
It is the amount of energy needed to raise the temperature of 1 kg of a substance by 1°C.
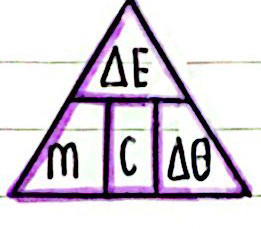
This depends on the material of the substance, its mass, and the energy added. The formula for calculating the change in thermal energy is: change in thermal energy = mass × specific heat capacity × temperature change
Where ∆E is the change in thermal energy, in joules J, specific heat capacity, c in joules per kilogram per degree Celcius , mass m in kilograms kg and temperature change ∆T in degrees Celcius °C.
Rearranged Formula:
Key Points
- Heating a substance increases its internal energy, which is the energy in its thermal store or the kinetic energy of its particles.
- In kinetic theory, temperature measures the average internal energy of the particles in a substance.
- Materials that require a lot of energy to heat up also release a lot of energy when they cool down.
- The temperature change of a substance is related to its specific heat capacity, which is the energy needed to raise the temperature of 1 kg of a substance by 1°C.
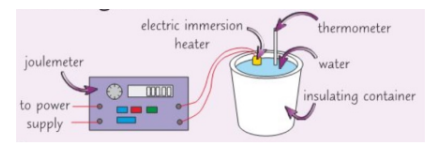
Finding the specific heat capacity of water/liquid
- Use a balance to measure the mass of the insulating container
- Set up an experiment (make sure joule metre reads zero & place a lid on the container)
- Measure the temp of the water then turn on the power
- Watch the thermometer as when it has increased by (e.g.. 10°C) stop the experiment and record the energy on the joule metre and increase in temperature
- You can then calculate the specific heat capacity of the water by rearranging the equation (do the experiment 3 times to get an average specific heat capacity)
500K+ Students Use These Powerful Tools to Master Specific heat capacity For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
10 flashcards
Flashcards on Specific heat capacity
Revise key concepts with interactive flashcards.
Try Physics Combined Science Flashcards1 quizzes
Quizzes on Specific heat capacity
Test your knowledge with fun and engaging quizzes.
Try Physics Combined Science Quizzes8 questions
Exam questions on Specific heat capacity
Boost your confidence with real exam questions.
Try Physics Combined Science Questions27 exams created
Exam Builder on Specific heat capacity
Create custom exams across topics for better practice!
Try Physics Combined Science exam builder28 papers
Past Papers on Specific heat capacity
Practice past papers to reinforce exam experience.
Try Physics Combined Science Past PapersOther Revision Notes related to Specific heat capacity you should explore
Discover More Revision Notes Related to Specific heat capacity to Deepen Your Understanding and Improve Your Mastery
Load more notes