Photo AI
Last Updated Sep 26, 2025
Kinetic Theory Simplified Revision Notes for GCSE Edexcel Physics
Revision notes with simplified explanations to understand Kinetic Theory quickly and effectively.
354+ students studying
3.3.1 Kinetic Theory
Gases and Pressure
The pressure, temperature, volume, and kinetic energy of a gas and its particles are interconnected. The particle model helps to explain these relationships and how altering one variable can impact the others.
Temperature and Pressure
Temperature and pressure in a gas are directly related. The particles in a gas are constantly moving in random directions with varying speeds. When the temperature of the gas is increased, the following occurs:
The increase in temperature results in a transfer of energy from heat to kinetic energy in the particles.
Kinetic energy and speed are related by the equation:
So, as kinetic energy increases, the speed of the particles also increases.
- Therefore, the average speed of the particles rises with temperature.
- With increased speed, gas particles collide more frequently, both with each other and with the walls of the container they are in.
- The pressure exerted by the gas is the total force applied by all the individual particles on the area of the container, which can be expressed as:
- Hence, an increase in temperature leads to an increase in the kinetic energy and speed of the particles, which in turn increases the pressure of the gas.
- This relationship holds true only if the volume of the gas remains constant.
Pressure and Volume
When the temperature of a gas remains constant, pressure and volume are inversely proportional. This means that as one increases, the other decreases. For example, if the volume of a gas is increased, its pressure will decrease because the gas particles are more spread out, leading to fewer collisions with the container walls.
This inverse relationship can be expressed by the equation:
Where:
- is the pressure in pascals (Pa).
- is the volume in cubic metres
For instance, if the volume of a gas (with constant temperature) is doubled, its pressure must halve to maintain the constant relationship between pressure and volume.
Increasing the Pressure of a Gas
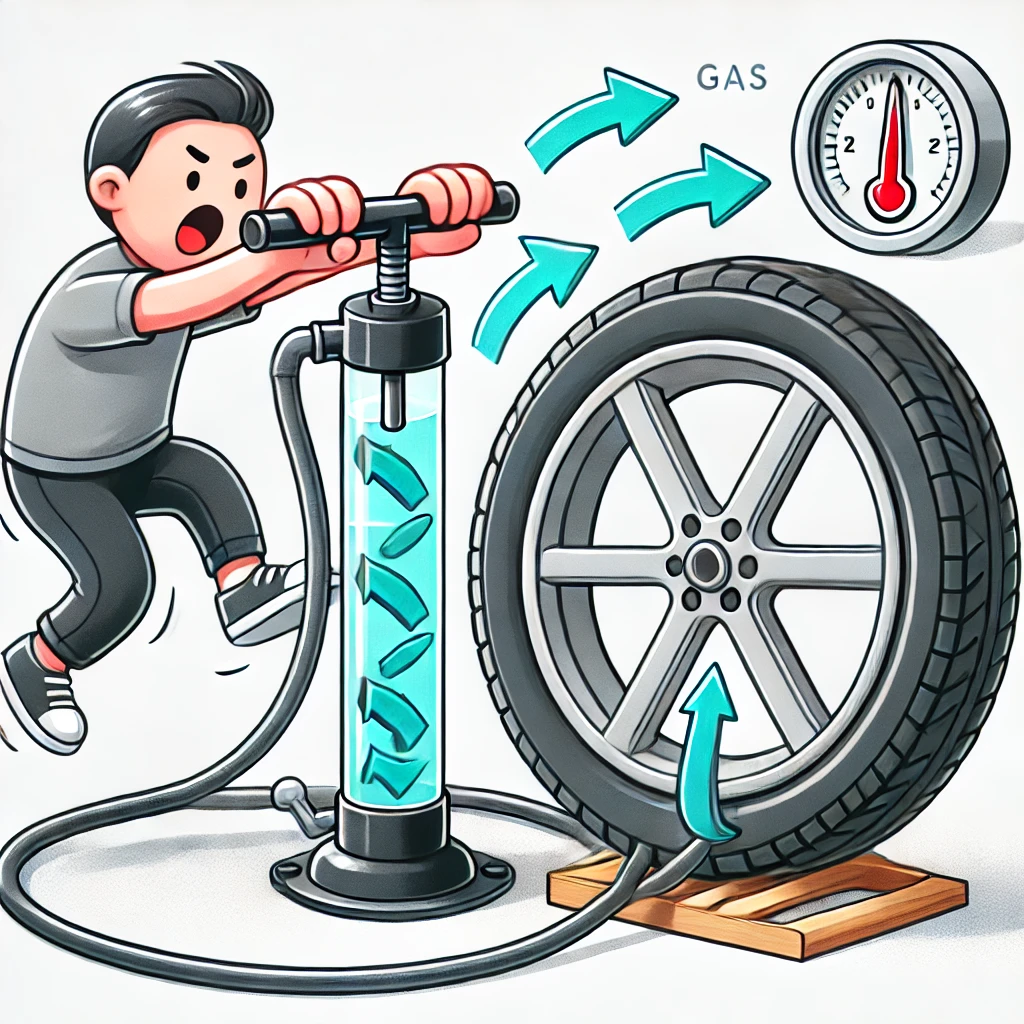
When energy is transferred by a force, this process is known as work. Doing work on a gas increases its internal energy, which, in turn, increases its temperature.
An example of this is using a bike pump. When you push down on the lever, the gas inside the pump exerts a force on the lever. By applying force to push the lever down, you do work against this force, increasing the internal energy of the gas. As a result, the temperature of the gas—and the tyre—rises as you pump more air into it.
500K+ Students Use These Powerful Tools to Master Kinetic Theory For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
20 flashcards
Flashcards on Kinetic Theory
Revise key concepts with interactive flashcards.
Try Physics Flashcards2 quizzes
Quizzes on Kinetic Theory
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes17 questions
Exam questions on Kinetic Theory
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Kinetic Theory
Create custom exams across topics for better practice!
Try Physics exam builder78 papers
Past Papers on Kinetic Theory
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Kinetic Theory you should explore
Discover More Revision Notes Related to Kinetic Theory to Deepen Your Understanding and Improve Your Mastery
Load more notes