Photo AI
Last Updated Sep 26, 2025
Atomic Structure Simplified Revision Notes for GCSE Edexcel Physics
Revision notes with simplified explanations to understand Atomic Structure quickly and effectively.
273+ students studying
4.1.1 Atomic Structure
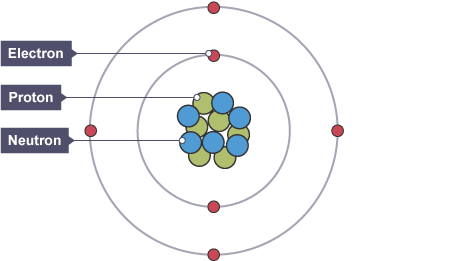
A positively charged nucleus (which contains neutrons and protons) is surrounded by negatively charged electrons.
| Subatomic Particle | Relative Mass | Relative Charge |
|---|---|---|
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | 0 (0.0005) | -1 |
Typical radius of an atom: metres
-
And the radius of the nucleus is 10,000 times smaller
-
Most (nearly all) of the mass of the atom is concentrated in the nucleus Electron Arrangement:
-
Electrons lie at different distances from the nucleus (different energy levels). The electron arrangements may change with the interaction with EM radiation.
Atomic Structure: Developing the Model of the Atom
Historical Development
- Greeks:
- Atoms are tiny spheres which cannot be divided.
- Thomson's Plum Pudding Model:
- An atom is a ball of positive charge with negative electrons embedded in it.
1909 - Rutherford's Alpha Scattering Experiment
- Fired a beam of alpha particles (positively charged) at a thin sheet of gold foil.
- Most particles went straight through → Atom is mostly empty space.
- Some were deflected → The Center of the atom must have a positive charge, repelling particles that come close to it.
- Some bounced straight back → Mass is concentrated in the centre.
- This research led to the development of the first nuclear model.
Bohr's Model
- Electrons orbit the nucleus at specific distances.
- Orbits are called energy levels or shells.
Updated Nuclear Model
- Positive Nucleus: Contains protons and neutrons.
- Electron Energy Levels: Electrons occupy energy levels or shells around the nucleus.
James Chadwick
- Proved the existence of the neutron which explained the imbalance between atomic and mass numbers.
Current Model of the Atom
- Nucleus: Contains protons and neutrons.
- Electrons: Occupy energy levels or shells around the nucleus.
Atomic Structure and Energy Levels
Subatomic Particles
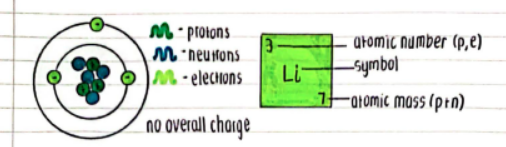
- Protons (p)
- Positive charge
- Neutrons (n)
- Neutral charge
- Electrons (e)
- Negative charge
- Overall Charge
- Atoms have no overall charge.
Atomic Representation
- Atomic Number (p, e):
- Number of protons and electrons in the atom.
- Atomic Mass (p + n):
- The sum of protons and neutrons in the atom.
- Symbol:
- Element symbol (e.g., Li for Lithium).
Energy Levels
- Higher Energy Levels:
- Energy levels further from the nucleus are at a higher energy.
- Absorption and Emission of Electromagnetic Radiation:
- If the atom absorbs electromagnetic radiation, an electron can move from a lower energy level to a higher energy level.
- The atom can then emit electromagnetic radiation, causing the electron to return to the lower energy level.
500K+ Students Use These Powerful Tools to Master Atomic Structure For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
20 flashcards
Flashcards on Atomic Structure
Revise key concepts with interactive flashcards.
Try Physics Flashcards2 quizzes
Quizzes on Atomic Structure
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes23 questions
Exam questions on Atomic Structure
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Atomic Structure
Create custom exams across topics for better practice!
Try Physics exam builder78 papers
Past Papers on Atomic Structure
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Atomic Structure you should explore
Discover More Revision Notes Related to Atomic Structure to Deepen Your Understanding and Improve Your Mastery
Load more notes