Photo AI
Last Updated Sep 26, 2025
Alcohol Production Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Alcohol Production quickly and effectively.
223+ students studying
Alcohol Production
Definition and Overview
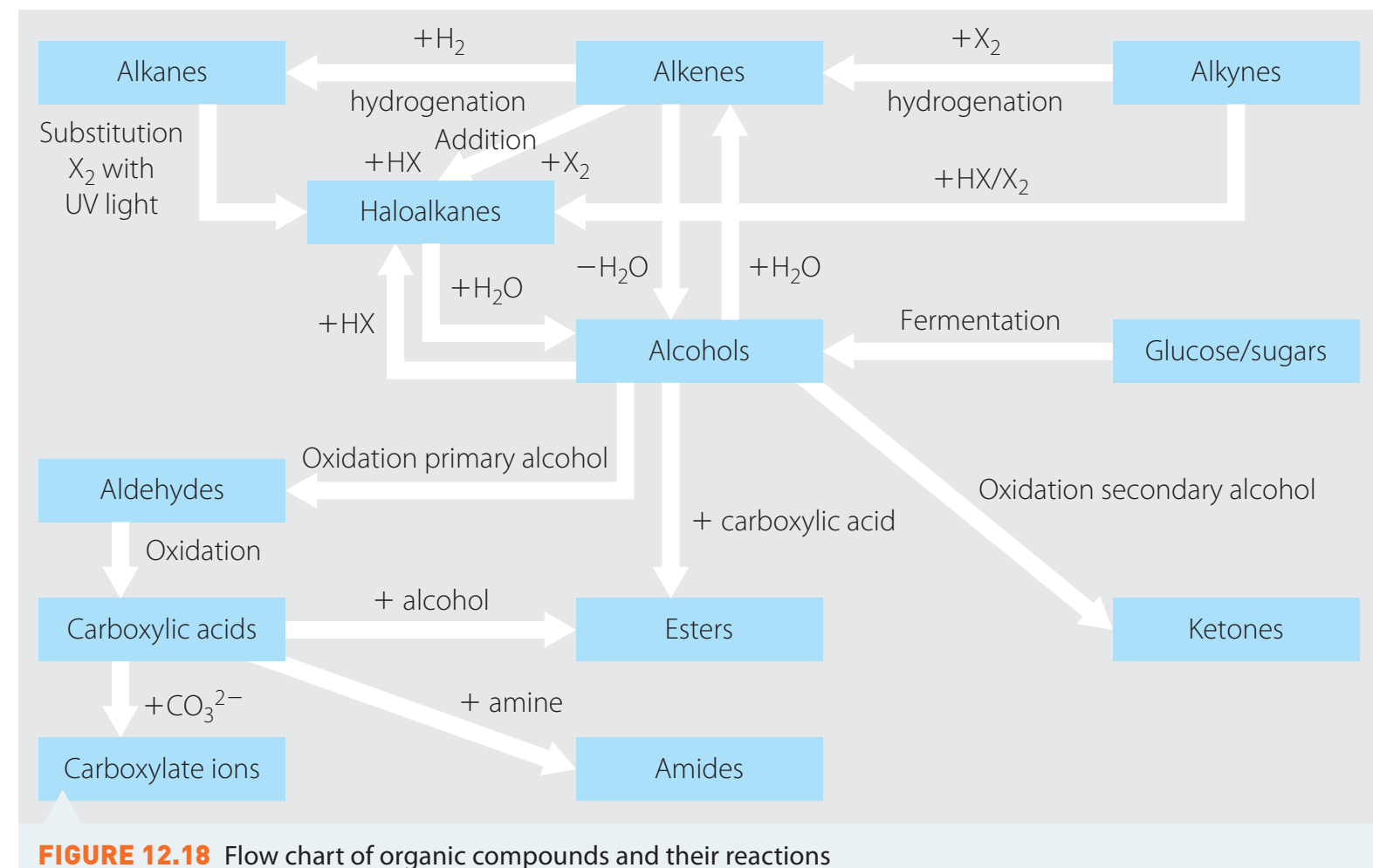
Introduction to Haloalkanes
- Haloalkanes (Alkyl Halides): Organic compounds consisting of one or more halogen atoms attached to a carbon atom.
- Significance: The polar carbon-halogen bond is particularly reactive due to the electronegativity difference between carbon and the halogen.
- Reactivity: The polarity facilitates nucleophilic attacks on the electron-deficient carbon atoms, essential for substitution reactions.
Haloalkane (R-X): Organic molecule where 'R' denotes an alkyl group and 'X' represents a halogen, such as Cl, Br, or I.
Nucleophile: An electron-rich entity that targets electron-poor sites.
Nucleophilic Substitution
- Role of Nucleophiles: These entities convert alkyl halides into alcohols by donating electrons.
- Example: Bromoethane reacts with a hydroxide ion to yield ethanol.
Step-by-Step Example:
- Initiation: The nucleophile approaches the electrophile (haloalkane).
- Nucleophilic Attack: Electrons are transferred, forming a new covalent bond.
- Leaving Group Departure: The halogen exits, resulting in the formation of an alcohol.
Substitution is facilitated by nucleophiles donating electrons to an electron-poor carbon in haloalkanes.
Types of Reactions
S1 and S2 Mechanisms
-
S1:
- Mechanism: Involves a carbocation intermediate in a unimolecular process.
- Favourable Conditions: Occurs with weak nucleophiles in polar protic solvents, typically with tertiary haloalkanes.
- Example: Conversion of tert-butyl chloride to tert-butanol.
-
S2:
- Mechanism: Bimolecular and proceeds through a concerted single-step reaction.
- Favourable Conditions: Occurs with strong nucleophiles in polar aprotic solvents, usually with primary haloalkanes.
- Example: Transformation of methyl chloride with strong nucleophiles.
-

Reactivity Based on Halogen
Comparative Reactivity
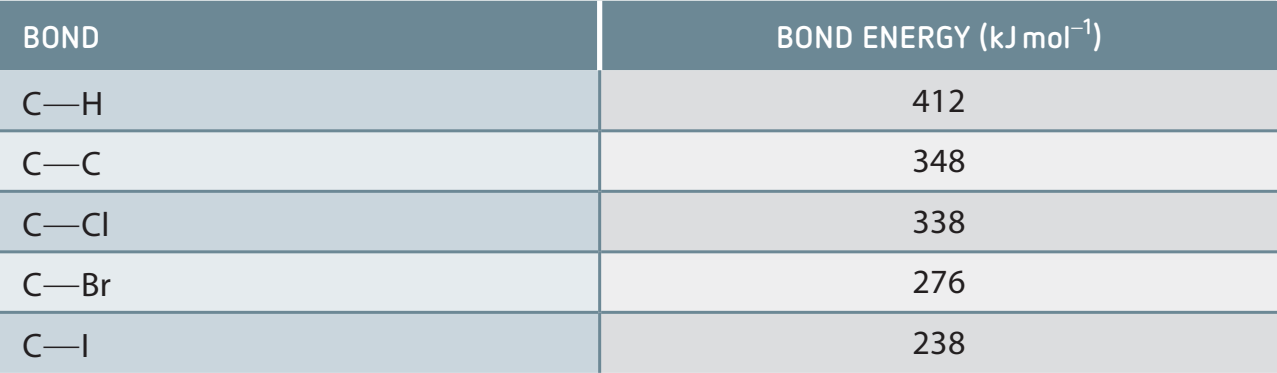
- Reactivity Order: Iodo > Bromo > Chloro
- Reason: Lower bond energy correlates with higher reactivity, which explains the high reactivity of iodine compounds.
Mechanism Choice By Nucleophile Strength
- Strong Nucleophile: Prefers S2.
- Weak Nucleophile: Prefers S1.
Reaction Conditions
Factors Affecting Reactions
-
Temperature: Elevated temperatures expedite reaction rates.
-
Solvents:
- Polar aprotic solvents promote S2 as they do not solvate the nucleophile.
- Polar protic solvents stabilise intermediates in S1 reactions.
-
Nucleophile Concentration: Higher concentrations favour S2 due to increased probability of bimolecular interactions.
-
Polar aprotic solvents, such as Dimethyl Sulfoxide (DMSO), enhance S2 reactions by avoiding nucleophile solvation, thereby accelerating the reaction.
Experimental Setup
Laboratory Methods
- Safety Procedures: Ensure laboratories are well-ventilated. Wear protective gloves and goggles.
- Necessary Glassware: Utilise distillation apparatus under moisture-free conditions.
- Example: Converting 2-bromobutane to 2-butanol.
Procedure:
- Preparation:
- Arrange a moisture-free distillation setup, ensuring all seals prevent volatile losses.
- Introduce reactants, carefully monitoring temperatures for effective substitution.
Haloalkanes are often volatile and toxic; adhere to all safety protocols.
Fermentation Process in Alcohol Production
Introduction
Fermentation: A metabolic process converting sugars into alcohols or acids. This process occurs without the presence of oxygen, in contrast to cellular respiration, which requires oxygen to produce energy. Fermentation allows organisms to metabolise energy in anaerobic environments.
-
Key Role:
- Facilitates energy (ATP) production in the absence of oxygen.
- Supports organism survival in anaerobic conditions.
-
Historical Context:
- Ancient Cultures:
- Utilised for transforming milk into yoghurt, dough into bread.
- Played a vital role in food preservation and alcohol production.
- Modern Applications:
- Essential for biofuels and pharmaceuticals.
- Fosters advancements in biotechnology, vaccines, and diagnostics globally.
- Ancient Cultures:
Biochemical Pathway
Overview of Enzymatic Pathways
-
Glycolysis:
- Transforms glucose into pyruvate, producing energy in the form of 2 ATP molecules.
- Key reactions and enzymes:
- Hexokinase: Initiates energy extraction from glucose through phosphorylation.
- Aldolase: Cleaves fructose 1,6-bisphosphate, advancing energy-releasing reactions.
-
Alcoholic Fermentation:
- Converts pyruvate into ethanol and CO.
- Enzymes involved:
- Pyruvate Decarboxylase: Converts pyruvate into acetaldehyde.
- Alcohol Dehydrogenase: Converts acetaldehyde into ethanol, regenerating NAD.
- Key Reactions:
- Metabolic Fate:
- Post-fermentation, ATP supports cellular activities.
- NAD recycling ensures continued glycolysis.
500K+ Students Use These Powerful Tools to Master Alcohol Production For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
193 flashcards
Flashcards on Alcohol Production
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards23 quizzes
Quizzes on Alcohol Production
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes269 questions
Exam questions on Alcohol Production
Boost your confidence with real exam questions.
Try Chemistry Questions10 exams created
Exam Builder on Alcohol Production
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Alcohol Production
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Alcohol Production you should explore
Discover More Revision Notes Related to Alcohol Production to Deepen Your Understanding and Improve Your Mastery
