Photo AI
Last Updated Sep 24, 2025
Alcohols - Dehydration Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Alcohols - Dehydration quickly and effectively.
300+ students studying
Alcohol Dehydration
Introduction
Understanding alcohol dehydration reactions is vital in organic chemistry. Dehydration involves the removal of water from alcohols to form alkenes. This process is fundamental for producing petrochemicals and has significant industrial and educational importance. Alkenes are essential in manufacturing consumer products like cling films and car tyres.
Essential Role: Alcohol dehydration reactions are key for producing numerous everyday items such as cling films and car tyres.
Key Terms and Definitions
-
Alcohol: A compound containing a hydroxyl (-OH) group, with variations across primary, secondary, and tertiary structures.
-
Dehydration: The chemical process of removing water to facilitate molecular transformations.
-
Alkene: Hydrocarbons with a carbon-carbon double bond, important in the production of basic materials and chemicals.
-
Reactivity: Measurement of a substance's propensity to undergo a chemical reaction.
-
Carbocation: Positively charged ion, important in reaction intermediates.
Introduction to Dehydration Reaction
Dehydration reactions are fundamental in organic chemistry, converting alcohols into alkenes by removing water molecules. For instance, ethanol is converted to ethylene, a critical component in plastic production. Similarly, cyclohexanol can be dehydrated into cyclohexene.
Industrial Impact: Annually, over 150 million tonnes of ethylene are produced worldwide, highlighting the extensive influence of dehydration reactions.
Explanation of the Mechanism
General Dehydration Reaction Process
This reaction involves several steps transforming an alcohol into an alkene and water. A typical conversion is illustrated below:
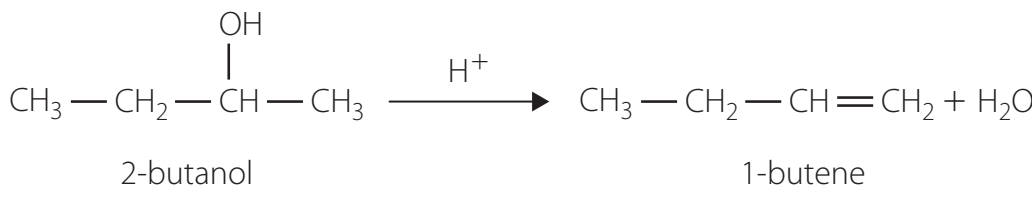
-
Step 1: Protonation
- Transforms the hydroxyl group into a more effective leaving group, facilitated by catalysts like sulphuric and phosphoric acids.
- Protonation initiates the reaction by converting the alcohol into a reactive species.
-
Step 2: Formation of Carbocation
- Water is eliminated, resulting in a carbocation.
- Stability ranking: Tertiary > Secondary > Primary.
-
Step 3: Elimination to Form Alkene
- Removal of a proton leads to the formation of a double bond.
Carbocation stability affects the reaction's direction and efficiency.
Worked Examples
Example: Ethanol Dehydration
- Ethanol is protonated by acid catalyst (H⁺)
- Water molecule leaves, forming a carbocation
- A proton is removed from an adjacent carbon, creating ethene
- The reaction can be summarised as: CH₃CH₂OH → CH₂=CH₂ + H₂O
Example: Cyclohexanol Dehydration
- Acid catalyst protonates the hydroxyl group
- Water leaves, forming a cyclic carbocation
- Deprotonation results in cyclohexene
- Overall: C₆H₁₁OH → C₆H₁₀ + H₂O
Factors Affecting Dehydration Reactions
Alcohol Structure and Reactivity
- Primary Alcohols: Least reactive due to their least stable carbocations.
- Secondary Alcohols: Exhibit moderate reactivity with relatively stable carbocations.
- Tertiary Alcohols: Highly reactive, forming the most stable carbocations, which results in rapid reactions.
| Alcohol Type | Reactivity | Carbocation Stability |
|---|---|---|
| Primary | Low | Least stable |
| Secondary | Moderate | Moderately stable |
| Tertiary | High | Most stable |
Catalysts
- Acid Catalysts: Sulphuric and phosphoric acids accelerate reaction rates.
- Alternative Catalysts: Aluminium oxide offers different catalytic effectiveness.
Catalysts are crucial as they lower activation energy, thereby speeding up the reaction.
Reaction Conditions
- Temperature: Higher temperatures (170-180°C) generally enhance reaction rates and product yield.
- Catalyst Concentration: Impacts the rate and efficiency of reactions.
Elevated temperatures are essential for reaction efficiency in industrial applications.
Impact Table
- Temperature and Reaction Rates:
General Equation for Alcohol Dehydration
Chemical Equation
- General Equation: Alcohol + Catalyst → Alkene + Water
- Example with Ethanol:
Role of Catalysts
- Acid Catalysts: Sulphuric Acid (H₂SO₄) or Phosphoric Acid (H₃PO₄) reduce activation energy.
- Alternative Catalyst: Aluminium oxide provides varying catalytic benefits.
Exam Tips: Understand the roles of catalysts in reaction dynamics.
Predicting Products of Dehydration
Saytzeff's Rule
- Significance: Predicts the major alkene product, underscoring its importance in exams.
- Rule: The alkene with the most substitutions is typically the major product.
Saytzeff's Rule: The more substituted alkene tends to be the major product.
Examples
- Example: 2-Butanol Dehydration
- Reactant: CH₃CH₂CH(OH)CH₃
- Products: 1-butene and 2-butene.
- Major Product: 2-butene, following Saytzeff's Rule.
Safety Measures in Dehydration Reactions
Key Lab Apparatus
- Distillation Setup: Vital for conducting dehydration reactions effectively.
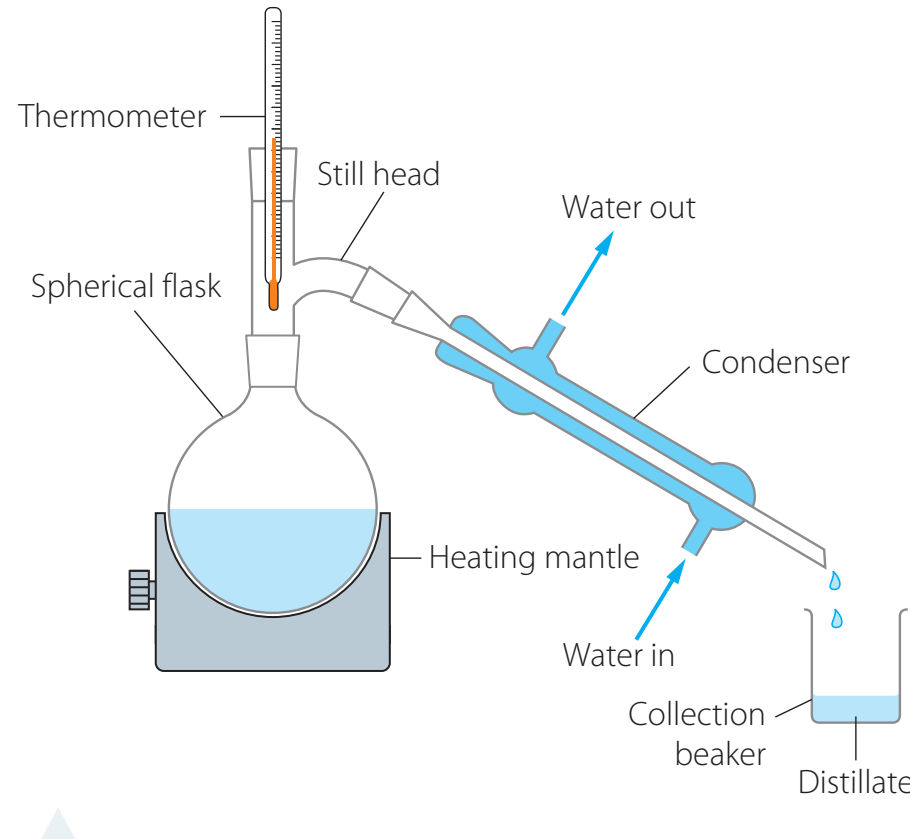
- Inspection Checklist:
- Glassware Integrity: Check for any cracks or defects.
- Ensure all connections are tightly sealed.
Never use glassware that is damaged.
Essential Safety Procedures
-
Handling Concentrated Acids:
- Use of PPE is mandatory: gloves, goggles, and lab coats.
- Employ fume hoods to minimise exposure.
-
First Aid:
- Rinse immediately in case of acid contact.
- Ensure availability of first-aid kits and eyewash stations in the lab.
Practice Questions with Solutions
-
Write the balanced chemical equation for ethanol dehydration.
- Solution: C₂H₅OH → C₂H₄ + H₂O
-
Compare phosphoric acid vs. aluminium oxide as catalysts.
- Solution: Phosphoric acid (H₃PO₄) is a homogeneous catalyst that provides hydrogen ions for protonation. Aluminium oxide (Al₂O₃) is a heterogeneous catalyst that works at high temperatures and can be more easily separated from products.
-
Apply Saytzeff's Rule to predict the products of 2-butanol dehydration.
- Solution:
- Possible products: 1-butene (CH₃CH₂CH=CH₂) and 2-butene (CH₃CH=CHCH₃)
- Major product: 2-butene, as it has the more substituted double bond (two alkyl groups attached to the C=C)
- Minor product: 1-butene, as it has a less substituted double bond (one alkyl group attached to the C=C)
- Solution:
Conclusion
Thorough understanding of alcohol dehydration reactions is essential for exam success. Consistent and varied practice is necessary to enhance analytical skills and readiness for examinations. Engage with diverse question formats and strengthen comprehension of catalysts, reaction conditions, and molecular structure dynamics.
Regular practice helps identify knowledge gaps and facilitates targeted improvements.
500K+ Students Use These Powerful Tools to Master Alcohols - Dehydration For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
193 flashcards
Flashcards on Alcohols - Dehydration
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards23 quizzes
Quizzes on Alcohols - Dehydration
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes269 questions
Exam questions on Alcohols - Dehydration
Boost your confidence with real exam questions.
Try Chemistry Questions10 exams created
Exam Builder on Alcohols - Dehydration
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Alcohols - Dehydration
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Alcohols - Dehydration you should explore
Discover More Revision Notes Related to Alcohols - Dehydration to Deepen Your Understanding and Improve Your Mastery
