Photo AI
Last Updated Sep 24, 2025
Chemistry - Alcohols Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Chemistry - Alcohols quickly and effectively.
356+ students studying
Chemistry - Alcohols
Overview of Alcohols
Alcohols: Organic compounds characterised by hydroxyl (-OH) groups attached to saturated carbon atoms. These functional groups significantly influence their chemical behaviour:
- Boiling points
- Solubility
- Reactivity
These properties are largely due to hydrogen bonding.
Key Term: Saturated Carbon Atoms refer to carbon atoms connected through single bonds, enabling maximum hydrogen attachment.
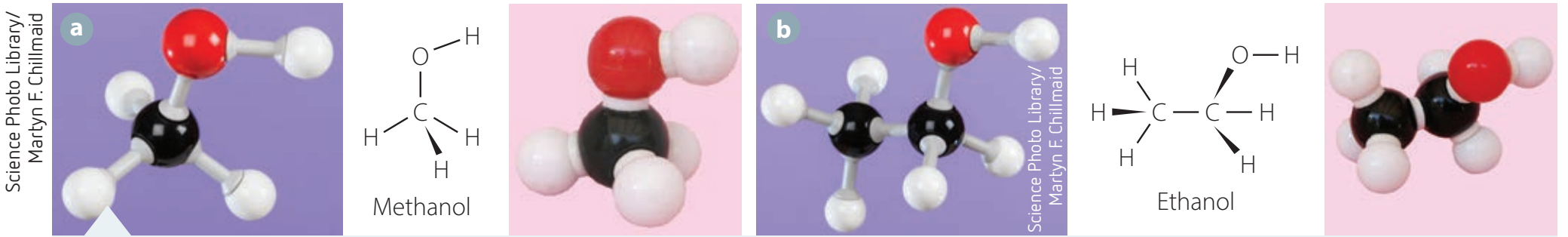
Examples of Common Alcohols
Alcohols vary widely in structure and uses:
- Methanol (CH₃OH):
- Exhibits a simple structure.
- Predominantly toxic, yet crucial in industrial applications.
- Ethanol (C₂H₅OH):
- Integral to beverages, fuels, and as a solvent, owing to its balanced characteristics.
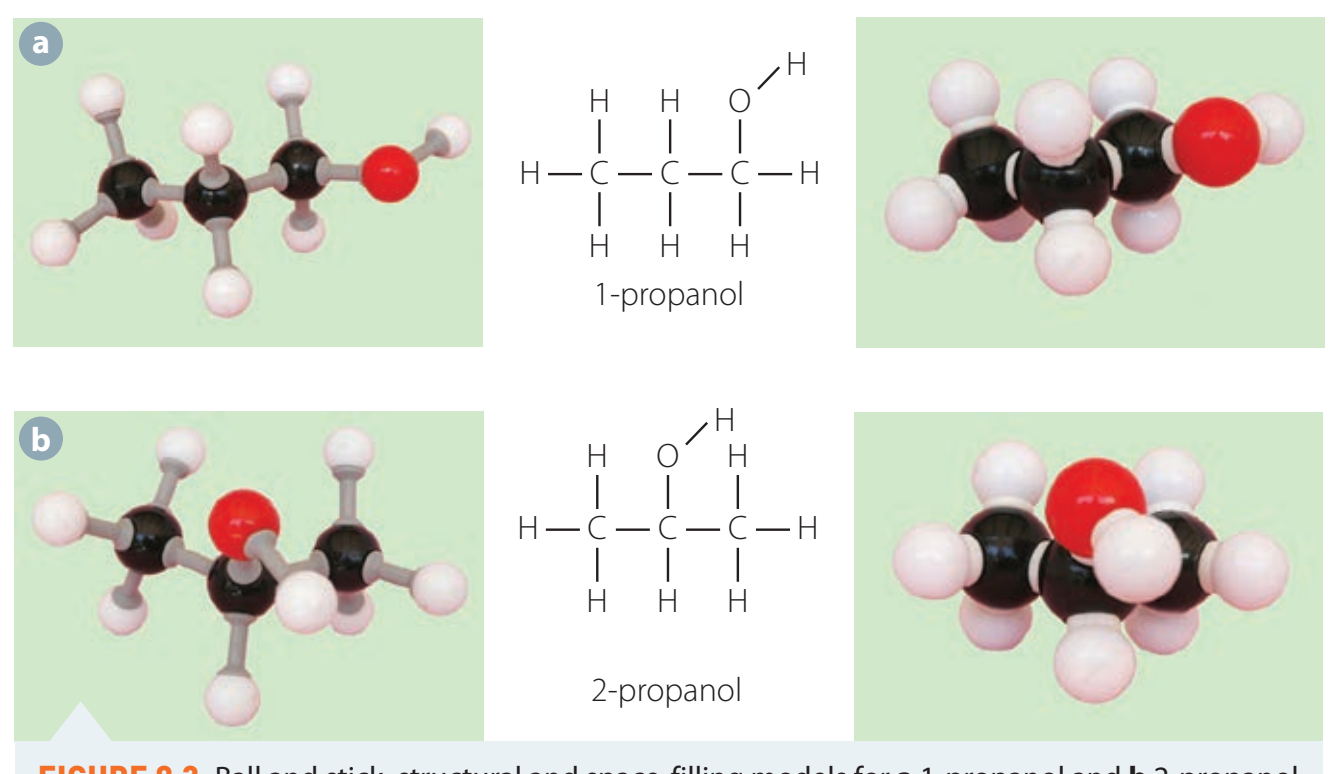
- Propanol (C₃H₇OH):
- Exists in two isomers; valuable in cleaning products and laboratories.
- Its structure allows for diverse uses.
Molecular Formulas and Structures
Grasping these structures is essential for comprehending their properties and practical applications.
Importance of Alcohols in Combustion Reactions
Alcohols are renewable and eco-friendly energy sources. They offer:
- Reduced pollution compared to hydrocarbons.
- Deliver a consistent energy output, making them ideal for studies and practical applications.
Introduction to Enthalpy and Combustion
Enthalpy: An essential measurement of heat transformation in chemical processes.
Key Points:
- Crucial for assessing energy efficiency.
- Vital to understanding reaction completion.
In combustion reactions, enthalpy elucidates energy release.
Did You Know? Ethanol serves a dual purpose in fuels and alcoholic beverages. This dual functionality shows its versatility and impact on industry and daily living.
Introduction to Combustion
- Combustion: A chemical process involving an exothermic reaction that produces carbon dioxide (CO₂) and water (H₂O).
- Significance: Knowledge of alcohol combustion is vital for studying energy production and efficiency.
General Balanced Equation
-
General Formula for the combustion of alcohols:
-
Components:
- Alcohol (CnH2n+1OH): Acts as the reactant.
- Oxygen (O2): A critical reactant for combustion.
- Products: Carbon dioxide (CO₂) and water (H₂O) are typically produced.
Specific Examples of Alcohol Combustion
| Alcohol | Formula | Balanced Equation |
|---|---|---|
| Methanol | CH₃OH | |
| Ethanol | C₂H₅OH |
- Stoichiometry: Balancing utilises stoichiometry to ensure the equality of atom types on both sides of the equation.
Role of Oxygen in Combustion
-
Complete Combustion:
- Yields only CO₂ and H₂O.
-
Incomplete Combustion:
- May result in CO, soot, or additional by-products.
Limited oxygen leads to incomplete combustion, causing less efficient and more polluting reactions.
Balancing Chemical Equations Step-by-Step
- Steps to Balance:
- Start with balancing carbon atoms.
- Continue with hydrogen atoms.
- Conclude with oxygen atoms, ensuring a balanced conservation of atoms.
Call-Out: Importance of Stoichiometry
Stoichiometry: Fundamental in predicting the result of combustion and ensuring environmental compliance.
- Accurate stoichiometric calculations are imperative.
Practice Problems
-
Challenge 1: Balance the combustion equation for propanol.
- Solution:
-
Challenge 2: Determine and balance the combustion reaction for pentanol.
- Solution:
- Step-by-step:
- Balance carbon: 5 carbon atoms on both sides
- Balance hydrogen: 12 hydrogen atoms total (11 + 1 from OH), giving 6H₂O
- Balance oxygen: 1 oxygen from pentanol + 15 from O₂ equals 16 total (10 in CO₂ + 6 in H₂O)
- Therefore, we need 7.5 molecules of O₂
- Common error: Forgetting to include the oxygen from the alcohol when counting total oxygen atoms
This guide encapsulates the core elements of alcohol combustion reactions. Mastering these concepts facilitates predicting reaction results and understanding their real-world implications.
Conditions Required for Combustion
Importance of Oxygen in Combustion
-
Oxygen: Fundamental for enabling combustion processes.
- Complete Combustion: Achieved with ample oxygen, producing carbon dioxide (CO₂) and water (H₂O).
- Incomplete Combustion: Occurs when oxygen is insufficient, resulting in carbon monoxide (CO) and soot.
-
Real-life Applications:
- Automobile Engines: Ensure complete combustion to optimise efficiency and safety.
- Domestic Heating Systems: Demand oxygen for secure energy production and mitigating carbon monoxide risks.
Incomplete combustion results in hazardous gases, posing safety risks.
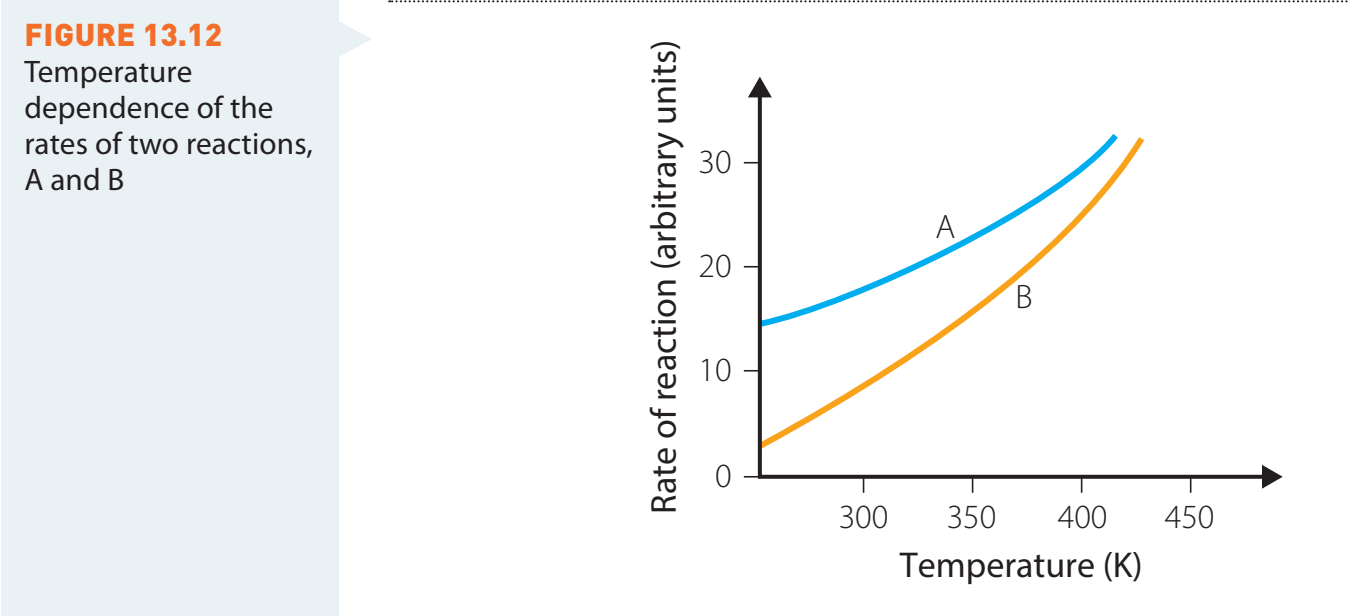
Temperature Requirements for Combustion
-
Ignition Temperature: The minimal temperature necessary for alcohol ignition.
- Methanol ignites at approximately 11°C.
- Ethanol ignites at nearly 13°C.
-
Significance:
- Knowledge of these temperatures is essential for safety and efficiency, influencing both fuel selection and material handling.
Different alcohols require distinct ignition temperatures.
Role of Catalysts
-
Catalysts: Compounds that reduce activation energy, facilitating combustion.
- Example: Platinum is regularly used in laboratories to accelerate alcohol combustion.
-
Economic Advantages:
- Reduces energy consumption, leading to cost savings and promoting sustainability.
Laboratory Settings for Combustion Studies
-
Typical Setup:
- Comprises a spirit burner, a calorimeter, and an oxygen supply system.
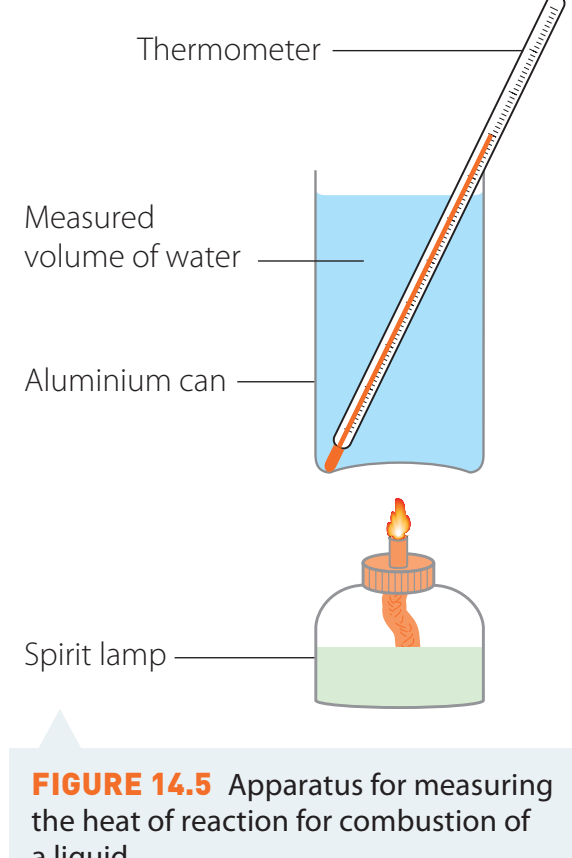
- Comprises a spirit burner, a calorimeter, and an oxygen supply system.
-
Safety Protocols:
- Ensure adequate ventilation to prevent gas accumulation.
- Steps to address equipment malfunctions:
- Turn off the burner.
- Allow equipment to cool down.
- Consult with an instructor if problems persist.
Consequences of Incomplete Combustion
-
Repercussions:
- Creates toxic gases such as carbon monoxide.
- Diminished energy efficiency.
-
Indicators:
- A "clean flame" appears blue and devoid of smoke.
- Signifies complete combustion.
- A "clean flame" appears blue and devoid of smoke.
Ensuring complete combustion is crucial to avoid the accumulation of hazardous gases and to maximise energy output.
Clarifying Visuals
- Diagrams portray:
- Reaction pathways for complete versus incomplete combustion:
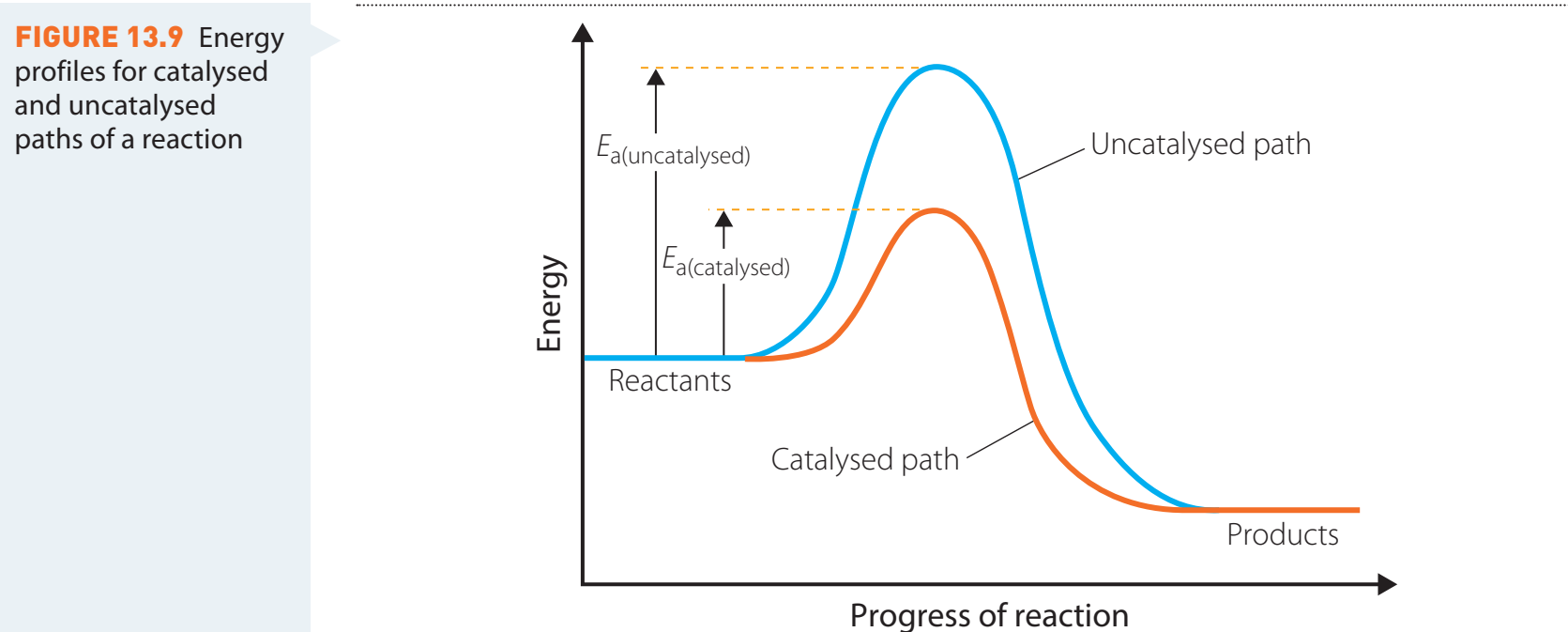
- Temperature vs. combustion graph:
- Reaction pathways for complete versus incomplete combustion:
Overview
- The goal is to determine the enthalpy change of combustion for various alcohols. This analysis offers insights into the energy content and efficiency of different fuels.
Understanding the purpose of this study is crucial, as it emphasises the vital connection between the energy content of fuels and their effectiveness.
Setup and Equipment
- Spirit burner: Utilised to combust alcohol, essential for discharging energy into the system.
- Calorimeter: A metal container used to heat water, enabling precise measurement of temperature change.
- Thermometer: An essential device for monitoring temperature; it should be immersed in the water to ensure accurate readings.
- Using a stand and insulation is imperative to reduce heat loss and enhance precision.
Safety Measures
- Exercise caution with flammable liquids to avert incidents, and store them properly.
- Wear safety goggles and gloves to guard against possible splashes and emissions.
- Ensure ample ventilation in the laboratory to effectively disperse noxious gases.
Experimental Procedure
- Record the initial mass of the spirit burner with alcohol.
- Pour a measured volume of water into the calorimeter.
- Record the initial water temperature using the thermometer.
- Ignite the alcohol and allow it to heat the water.
- Measure the final temperature once combustion concludes.
- Weigh the remaining spirit burner to assess the alcohol consumed.
Data Collection
- Executing repeated trials is vital for enhancing accuracy and result validation.
- Diligently record data:
- Temperature changes
- Quantity of alcohol expended
- Rigorously maintain control variables such as consistent water volume and starting temperature.
Calculations
- Heat energy formula:
- : mass of water
- : specific heat capacity of the water
- : temperature change
- Molar enthalpy formula:
- : moles of alcohol consumed

Understanding is important as it quantifies the energy transferred during combustion.
Emphasis on Accuracy and Error Management
- Common sources of error entail heat dissipation to the environment, imprecise initial temperature readings, and evaporation losses.
- Strategies to ensure accuracy:
- Implement proper insulation for the calorimeter to minimise heat loss.
- Utilise high-precision thermometers and scales for accurate data collection.
Results and Interpretation
- Plot temperature change against the mass of alcohol burned to identify data trends and anomalies.
- Note and interpret any discrepancies or unexpected results—they may provide insights or highlight areas for methodological refinement.
Conclusion for this Experiment
- This experiment illustrates the practical application of thermochemistry and enriches the understanding of energy dynamics in combustion reactions.
- Example Scenario: Calculate the enthalpy change when 0.5 moles of ethanol are burned, producing 2500 J of energy.
- Solution:
- Example Question: Identify variables that should be controlled to accurately determine enthalpy change and explain the importance of achieving stability.
- Solution: Variables to control include water volume, initial temperature, insulation quality, and ambient conditions. Stability is important because it ensures that all energy from combustion is transferred to the water, minimising heat loss to surroundings and producing reliable, reproducible results.
Introduction
The enthalpy of combustion is a crucial concept in assessing the energy efficiency of chemical reactions, particularly alcohols. Understanding the energy released provides insights into their fuel potential.
The enthalpy of combustion carries significant implications, notably in the formulation of biofuels, crucial for mitigating carbon emissions.
Key Concepts
- Enthalpy of combustion: The energy change associated with the combustion of one mole of a substance in oxygen.
- Molecular structure: Affects energy release. Longer carbon chains typically release more energy.
Molecular structure: Configuration of atoms in a molecule.
- Example: Methanol, with one carbon, releases less energy than Butanol, which has four carbons.
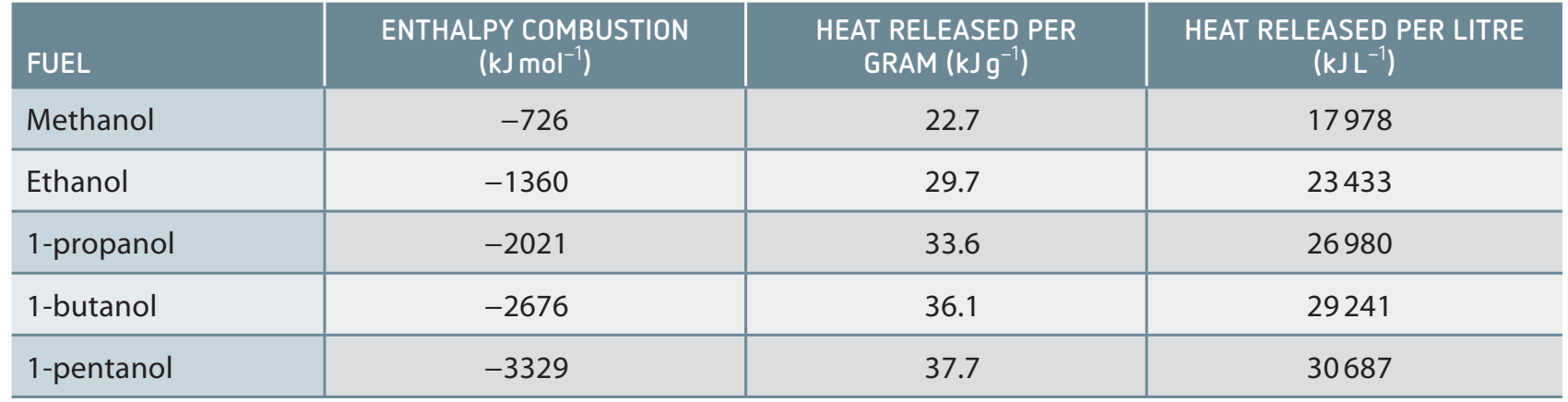
Data Presentation
Here's a table showing enthalpy values:
| Alcohol | Molecular Formula | Measured Enthalpy (kJ/mol) | Theoretical Enthalpy (kJ/mol) | Percentage Error (%) |
|---|---|---|---|---|
| Methanol | CHOH | -726 | -715 | 1.54 |
| Ethanol | CHOH | -1371 | -1367 | 0.29 |
| Propanol | CHOH | -2021 | -2010 | 0.55 |
| Butanol | CHOH | -2676 | -2660 | 0.60 |
Calculating Percentage Error: For methanol:
Analysis and Discussion
- Trend Analysis:
- Observations indicate that longer carbon chains result in increased energy release.
- Discrepancies:
- Arise from heat loss and data inaccuracies.
- Example: Methanol's 1.54% error indicates minor deviations potentially due to heat dissipation or measurement inaccuracies.

Conclusion of Analysis
The link between alcohol structure and the energy released is evident. Longer carbon chains emit more energy, beneficial for developing fuels.
Practical Implications: Understanding these correlations helps optimise fuel choices, contributing to eco-friendly solutions, as detailed in the introduction.
This comprehension aids in choosing appropriate alcohols for applications requiring efficient energy release, thereby advancing cleaner and more effective fuel technologies.
500K+ Students Use These Powerful Tools to Master Chemistry - Alcohols For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
193 flashcards
Flashcards on Chemistry - Alcohols
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards23 quizzes
Quizzes on Chemistry - Alcohols
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes269 questions
Exam questions on Chemistry - Alcohols
Boost your confidence with real exam questions.
Try Chemistry Questions10 exams created
Exam Builder on Chemistry - Alcohols
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Chemistry - Alcohols
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Chemistry - Alcohols you should explore
Discover More Revision Notes Related to Chemistry - Alcohols to Deepen Your Understanding and Improve Your Mastery
