Photo AI
Last Updated Sep 24, 2025
Mixtures in Chemistry Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Mixtures in Chemistry quickly and effectively.
453+ students studying
Mixtures in Chemistry
Introduction to Mixtures
Mixtures are essential to chemistry and are frequently encountered in everyday life. They play an important role in various applications, influencing areas like environmental science and culinary arts. Consider your morning coffee or orange juice; these are commonplace mixtures illustrating this importance.
Grasping the concept of mixtures is vital not only in laboratory settings but also in broader scientific and industrial contexts.
Definition of Mixtures
Mixtures: These are combinations of two or more substances that do not chemically bond, allowing each component to maintain its own properties.
- Common examples of mixtures include:
- Air
- Salad
- Soda
- Coffee
- Orange juice
Below is a visual representation distinguishing mixtures from pure substances:
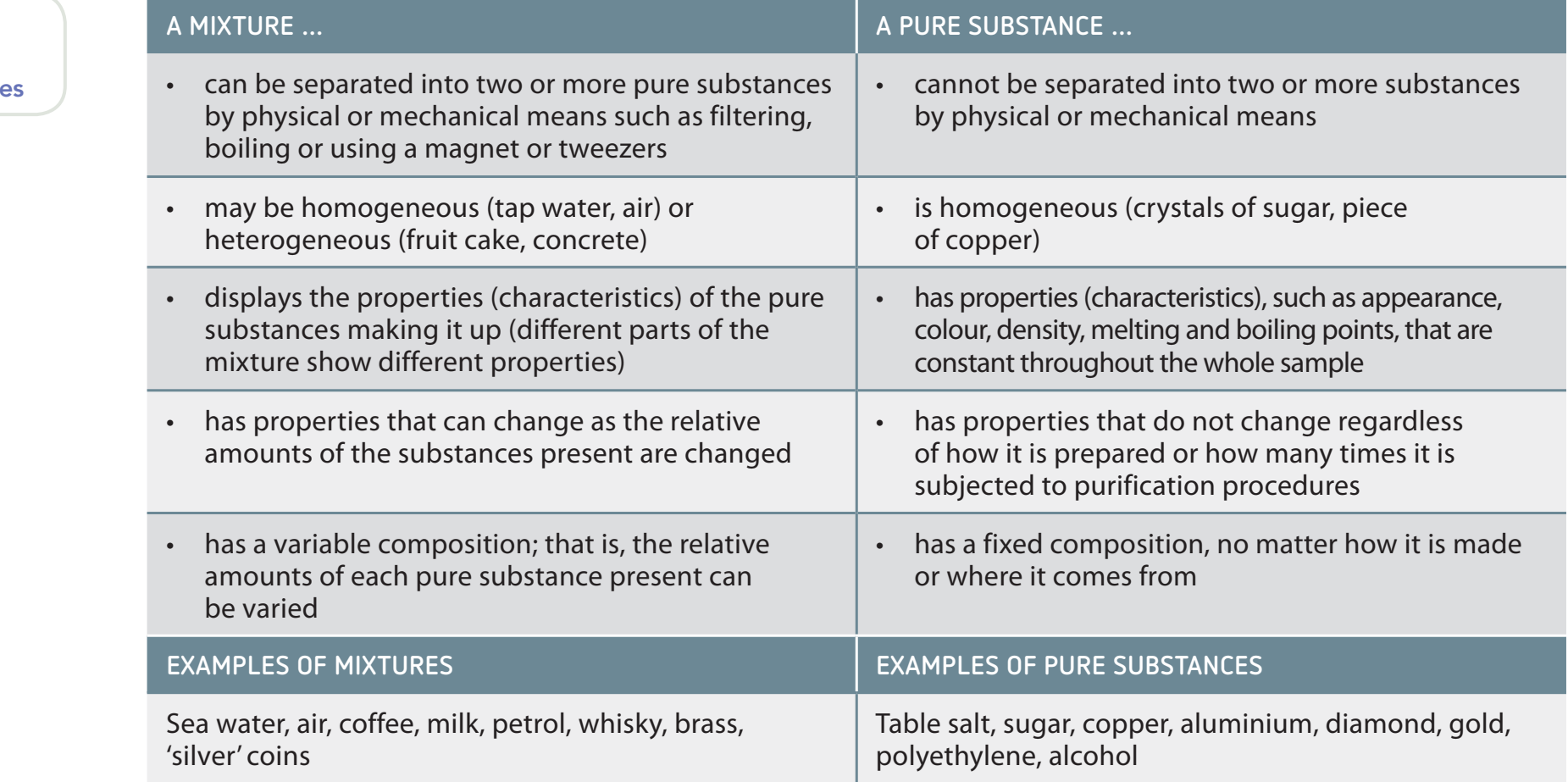
Key Distinction: Mixtures vs. Pure Substances
- Pure substances possess a uniform and definite composition, such as water (H₂O) and salt (NaCl).
- Mixtures vary in composition and can be separated by physical processes.
Properties of Mixtures vs. Pure Substances
Understanding the properties of mixtures and pure substances is paramount for predicting behaviour in chemistry and practical applications.
- Physical Properties: Characteristics such as melting point, boiling point, density, and solubility.
- Chemical Properties: Attributes like reactivity and the stability of the composition.
Comparative Analysis
- Melting and Boiling Points: Mixtures exhibit variable points due to their components. For instance, saltwater boils at a higher temperature than pure water.
- Solubility: Components in mixtures dissolve differently, such as how sugar dissolves in coffee, affected by factors like temperature.
- Density: A mixture's density, such as that of air, is an average of its components compared to the consistent density found in pure substances.
- Key Insight: Mixtures display variability due to the diversity of their components. Pure substances remain consistent in their behaviour, given all molecules are identical.
Classification of Mixtures
Homogeneous Mixtures
Homogeneous Mixtures: These have a uniform composition and occupy a single phase.
- Examples include:
- Saline water
- Air
- Vinegar
Characteristics
- Consistent appearance and composition throughout.
- Uniform consistency in pharmaceuticals ensures precise dosing.
Suggested Experiment:
- Dissolve salt in water and observe the formation of uniformity.
Heterogeneous Mixtures
Heterogeneous Mixtures: These have a non-uniform composition and consist of distinct phases.
- Examples include:
- Salads
- Granola
- Rocky road ice cream
Characteristics
- Visible distinct phases.
Practical Experiment:
- Combine oil and water; observe the separation of phases.
Emergent Properties and Applications of Mixtures
Emergent properties in mixtures are characteristics that appear only when individual substances interact, revealing features not evident in the individual components.
- Emergent Properties: Characteristics stemming from component interactions.
Examples include:
- Concrete: Enhanced compressive strength.
- Sugar Solutions: Altered boiling and freezing points.
- Alloys: Increased hardness and resistance.
- Colloids: Unique optical properties.
Practical Applications Across Industries
- Mining: Mixtures are instrumental in ore extraction.
- Food Production: Enhance flavour and preserve food products.
Separation Techniques
Choosing the Right Method
- Filtration: Isolates insoluble solids from liquids.
- Evaporation: Leaves the solute behind after liquid evaporation.
- Distillation: Separates substances based on different boiling points.
Filtration ensures samples are devoid of particulate contamination.
Safety Note: Always perform evaporation using suitable heat sources.
Percentage Composition by Weight
Percentage Composition by Weight: Mass percentage is a fundamental concept in chemistry. It connects theoretical understanding with practical applications.
Key Definitions and Formulas
-
Mass Percentage: The ratio of a component's mass to the total mass, represented as a percentage.
-
Calculation Formula:
Worked Example
Determine the salt percentage in a solution:
- Mass of Salt: 15g
- Total Mass of Solution: 150g
Exam Tips
- Carefully consider unit conversions during calculations.
- Recognise that emergent properties can affect the overall characteristics of mixtures.
- Familiarise yourself with key separation techniques to identify their appropriate applications.
500K+ Students Use These Powerful Tools to Master Mixtures in Chemistry For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
442 flashcards
Flashcards on Mixtures in Chemistry
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards40 quizzes
Quizzes on Mixtures in Chemistry
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes7 questions
Exam questions on Mixtures in Chemistry
Boost your confidence with real exam questions.
Try Chemistry Questions2 exams created
Exam Builder on Mixtures in Chemistry
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Mixtures in Chemistry
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Mixtures in Chemistry you should explore
Discover More Revision Notes Related to Mixtures in Chemistry to Deepen Your Understanding and Improve Your Mastery
